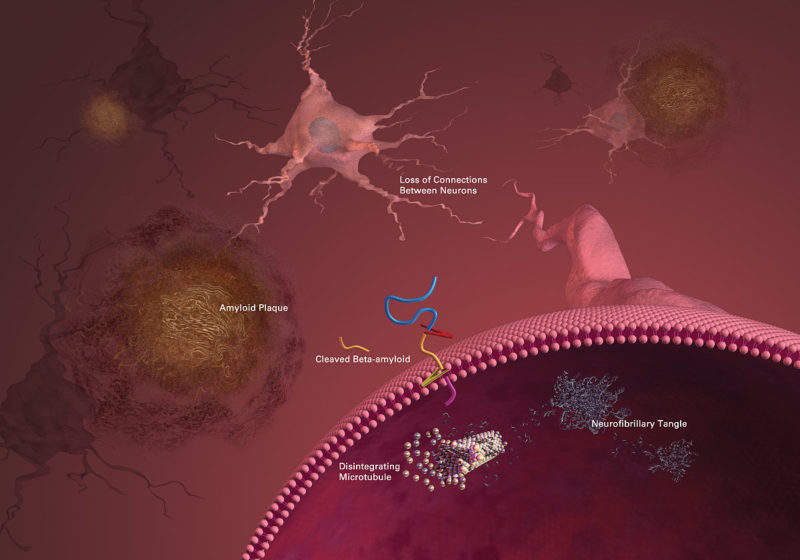
The long list of failures in the amyloid hypothesis casts doubts about the theory that the neurodegeneration in Alzheimer’s disease (AD) may be caused by deposits of amyloid β-peptide (Aβ) in plaques in brain tissue. A new drug, BAN2401, developed by Biogen and Japanese partner Eisai, showed a reduction in amyloid plaques and a slowing in the cognitive decline of AD patients.
At the 2018 Alzheimer’s Association International Conference, Biogen and Eisai presented the detailed results from the Phase II study (Study 201) with BAN2401, an Aβ inhibitor, which selectively binds to large, soluble Aβ protofibrils, in 856 patients with early AD. Study 201 (NCT01767311) was a placebo-controlled, double-blind, parallel-group, randomized Phase II clinical study in 856 patients with mild cognitive impairment (MCI) due to AD or early AD with confirmed amyloid pathology in the brain. The key primary endpoint, utilising Bayesian analysis of ADCOMS at 12 months, estimated the probability that the highest dose of BAN2401 slows clinical decline more than placebo was 98%. Compared to early success criteria at 12 months that was pre-specified as 80% or higher estimated probability of demonstrating a clinically significant difference (a 25% or greater slowing in clinical decline) from baseline compared to placebo, the actual probability for this criteria was 64%, according to Bayesian analysis.
The key secondary endpoint demonstrated a statistically significant dose-dependent slowing in cognitive decline from baseline on ADCOMS compared to placebo at 18 months. Other secondary measures also demonstrated statistically significant dose-dependent reduction in amyloid PET after 18 months, and acceptable tolerability profile was observed through the 18 months of treatment.
Positive result sparks cautious optimism
Some researchers have sparked cautious optimism and others expressed concerns about this new result, as initial data from Study 201, as announced in December 2017, showed BAN2401 did not meet the primary endpoint. However, the main difference between the negative primary outcome announced in December and the positive secondary outcome at 12 months is the statistical method employed: the former is based on Bayesian analysis, while the latter is derived from predefined conventional statistical method. Another possible reason for the success of the trial of BAN2401 is that it was one of the first to use PET scans to verify signs of Aβ in the brains of the study participants.
According to some researchers, the amyloid hypothesis has been the major explanation for the pathogenesis of AD for over 20 years, but all Aβ-targeting drugs to treat AD have ended in failure and recent studies indicated that the main factor concerning the development and progression of the disease is tau and not beta amyloid.
In 2018 alone, Merck, Eli Lilly/AstraZeneca, and J&J have discontinued late-stage trials of drugs designed to prevent the build-up of amyloid plaques in the brain, like BAN2401. However, pharma companies are still doing research in targeting Aβ, and Eisai and Biogen, in addition to amyloid beta antibody BAN2401, are co-developing BACE1 inhibitor E2609, also known as elenbecestat, currently in Phase III. The companies recently reported that the drug could lower Aβ levels in patients’ brains, although it did not significantly improve AD symptoms measured by CDR-SB in the same Phase II study in MCI and mild to moderate AD patients.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataWhile the long list of Phase III clinical trial failures has raised doubts about the approach and not all scientists are convinced that beta-amyloid is the primary cause of AD, the amyloid beta hypothesis remains central in the development of anti-AD drugs and the results showed by BAN2401’s Phase II clinical study are very encouraging.




Related Company Profiles
Biogen Inc