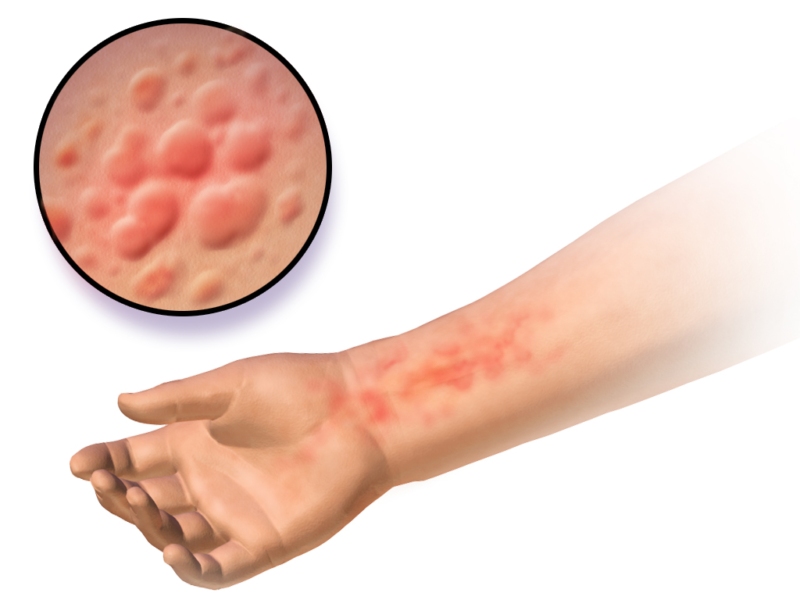
The first data on real-life adult atopic dermatitis patients being treated with Sanofi and Regeneron’s dupilumab was revealed at the proceedings of the 2018 European Academy of Dermatology and Venereology (EADV) Congress and consisted of two separate studies, one in the Netherlands over a period of 16 weeks and the other in France over a period of three months.
The former demonstrated that 85.3% of patients enrolled had achieved EASI-50 – a 50% improvement in symptoms based on the Eczema Area and Severity Index – 57.8% achieved EASI-75, and 22.5% achieved EASI-90. In comparison, the 2015 LIBERTY AD CHRONOS clinical trial had 80% reach EASI-50, 69% reach EASI-75, and 40% reach EASI-90; the 2017 LIBERTY AD CAFE clinical trial had 85%, 63%, and 46%, respectively.
Patient oriented eczema measure (POEM) scores also showed an increase of 91.8% compared to 77% from the CHRONOS trial and 84% from the CAFE trial. In the French study, 72% of patients achieved EASI-50 and 48.8% achieved EASI-75, while 52.8% achieved SCORAD-50 – a 50% improvement in symptoms based on the Scoring Atopic Dermatitis scale – and 16.6% achieved SCORAD-75. However, both studies concluded that many more real-life patients developed conjunctivitis than was recorded during clinical trials.
The Dutch prospective cohort study of 105 patients lasted for 16 weeks, during which biomarkers and EASI, investigator’s global assessment (IGA), and POEM scores were measured at screening and then every four weeks of treatment. The cytokines being measured were severity markers (such as IL-22, TARC), Th2 markers (such as IL-4 and IL-13), Th17 markers, Th22 markers, pruritus markers, eosinophil markers, and neutrophil markers and all markers were measured using Luminex-based multiplex immunoassays.
Of patients with previous use of systemic immunosuppressants, 37.1% had a history of at most one oral immunosuppressant treatment and 62.9% had a history of at least two oral immunosuppressants. The French multicentre retrospective cohort survey examined 241 patients in order to measure the median SCORAD at three months of dupilumab treatment and compare it to baseline SCORAD. The mean number of lines of systemic treatment prior to dupilumab was 2.9.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataDespite dupilumab’s strong efficacy profile, the number of ocular side effects was far greater in both of the real-life studies. The Dutch study saw 37% of patients develop allergic conjunctivitis, with 33% of those developing mild conjunctivitis and 67% developing moderate-severe conjunctivitis that needed anti-inflammatory treatment, and the French survey saw 38.2% of patients develop conjunctivitis. In comparison, only 8% of patients were reported as developing conjunctivitis in a meta-analysis of the SOLO1 and 2, CHRONOS, and Phase IIb trials of dupilumab. 10 patients (4.1%) in the French survey stopped dupilumab treatment because of the ocular side effects. Interestingly, dupilumab treatment had no effect on the biomarkers in the Th17 and Th22 pathways, or on neutrophil or pruritus markers.
A mechanism for the development of conjunctivitis during dupilumab treatment has not yet been confirmed but three possible mechanisms include:
- Increased activity of OX40 ligands involved in atopic keratoconjunctivitis
- Relatively less treatment of the eyes due to lower distribution of dupilumab in the tissues of the eyes
- Rosacea-like conjunctivitis caused by an increase in Demodex mites driven by IL-17 inflammation
New data presented at the 2018 EADV Congress demonstrated how palpebral conjunctiva biopsies taken from six patients who developed conjunctivitis while being treated with dupilumab had an increased CD4:CD8 cell ratio and a scarcity of intraepithelial goblet cells. A change in density of these specialised goblet cells has been associated with other ocular diseases. It was theorised that dupilumab-targeted disruption of IL-13 signaling decreased goblet cell mucin production, leading to tear film instability and causing epithelial barrier dysfunction which in turn could cause conjunctivitis.
In light of new evidence regarding dupilumab’s side effect profile, real-life patients should be warned of the likelihood of developing ocular side effects when placed on the treatment for atopic dermatitis, but the drug still has a favourable treatment profile when compared to systemic immunosuppressants for the treatment of moderate-severe atopic dermatitis.





Related Company Profiles
IGA, Inc.