A drug’s life cycle can last 20 to 30 years, and a single wrong move at any point along the way can cost millions if not billions of dollars.
Historically, the period in a drugs’ life cycle that was believed to determine its financial success was between initial approval and patent expiration – as the vast majority of a drug’s revenue is generated in this period.
However, with increased competition between pharmaceutical companies and ever-increasing scrutiny from regulators, healthcare payers and healthcare providers, it is becoming ever more important for drug developers to implement carefully considered life cycle management programs.
Development stage and regulatory approval
Taking a drug from early-stage development to the market is a lengthy and expensive process.
Preclinical development can take between one and six years, while the clinical development stage can take between six and 11 years, and the final new drug application can take between just over six months and two years.
Reducing the length of this development process is advantageous for drug developers both in terms of lower development costs, and also increasing the period of market exclusivity the drug enjoys.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataThis is because patents typically last only 20 years, so time spent developing the drug impacts the post-approval market exclusivity phase.
One way that companies can speed up the process is by making use of technology that helps complete clinical trials more rapidly.
3D medical imaging has advanced in the last decade, with companies such as Stryker Imorphics developing technology to improve the performance of anatomical modeling and speed up automated segmentation.
Market exclusivity phase
Market exclusivity is designed to encourage innovation and investment in the industry by protecting new products from competition.
A patent can be filed at any time during the development stage, which means that if a drug has a lengthy development process it is possible that it could reach the market with a very limited amount of patent protection remaining.
Therefore, to create a balance between innovation and the greater access to drugs that results from generic drug competition, the FDA awards five years of exclusivity following a successful new chemical entity application.
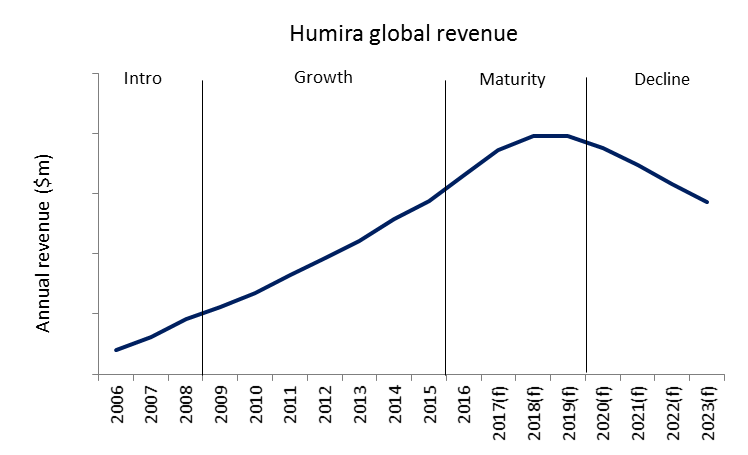
The market exclusivity phase is when companies attempt to recoup development costs and generate profit, and is commonly separated into three stages: introduction, growth and maturity.

Post-patent expiration
When a product’s patent or market exclusivity expires there are several strategies a company can use to fight generic completion to reduce revenue decline – although in the first instance most companies will instead look to extend the exclusivity period.
There are several strategies that can be implemented to achieve this, including claiming delays occurred during the initial application process both with the patent office and FDA; applying for pediatric exclusivity, which adds six months to existing marketing exclusivity; or applying for a new formulation patent, which provides less secure protection than the original product patent but can be successful in extending market exclusivity.



