China-based pharmaceutical outsourcing partner ShangPharma has signed a deal with the Qidong Biopharma Industrial Zone in Qidong, China, to build a biologics manufacturing and pre-clinical research facility.
The company will be the first tenant for the biopharma industrial zone.
ShangPharma comprises a family of companies, including China Gateway Biologics, China Gateway Pharmaceutical Development, ChemPartner, ShangPharma Technology and ShangPharma Investment.
The company will invest $60m in the project through a new subsidiary that will aim to provide and develop cutting-edge science and technology to expand ShangPharma’s biologics service portfolio.
China Gateway Biologics, the contract manufacturing wing of ShangPharma, will operate the manufacturing facility, while the pre-clinical research facility will be operated by ChemPartner, ShangPharma’s contract research wing.
The manufacturing and research facility is expected to be operational by early 2018. It aims to develop Qidong as China’s biotechnology hub and help ShangPharma to grow as a distinguished outsourcing and biopharmaceutical service provider.
Location of the ShangPharma facility in Qidong
The facility is being built in the Qidong Biopharma Industrial Zone in Lianyungang, Jiangsu Province.
Financing for the project
The project is being jointly financed by ShangPharma and the Qidong Biopharma Industrial Zone.
Details of ShangPharma’s manufacturing and pre-clinical research plant
The plant is aiming for high commercial-scale operations with an international focus, as well as ShangPharma’s overall biopharmaceutical development.
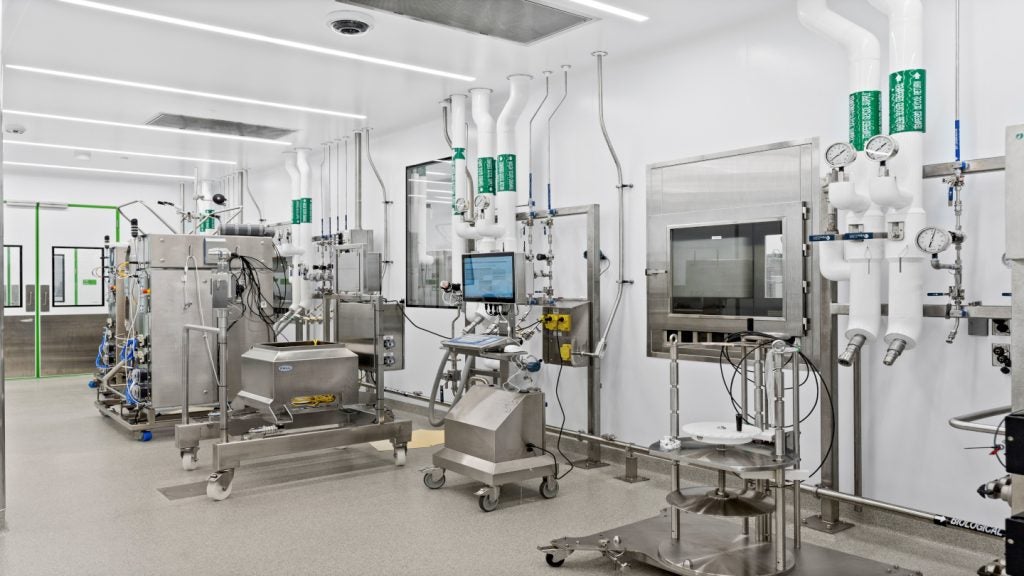
The facility will be equipped with a 500l single mammalian cell culture unit for clinical phase supply to be handled by ChemPartner, as well as two 2,000l units for commercial manufacturing operated by China Gateway Biologics.
The clinical supply and manufacturing units will be complemented with fill-and-finish capabilities of an appropriate scale.
Product portfolio of the ShangPharma plant
The investment is expected to grow China Gateway Biologics’ biopharmaceutical services by transforming it into a full biopharmaceutical service provider, from pre-clinical development to commercial-scale.
ChemPartner will be able to expand its clinical research abilities through the investment.
The state-of-the-art plant will comply with Western standards in order to support and meet the expectations of international clients.
Marketing commentary on ShangPharma, China Gateway Biologics and ChemPartner
ShangPharma is an outsourcing partner in pharmaceutical and biotechnology research and development, providing customised pharmaceutical, biotech, agrochemical, chemical, biology, biologics, and pre-clinical development services worldwide.
The company has established extensive research and development (R&D) partnerships within the healthcare industry and with organisations.
It also provides high-quality, cost-effective, integrated services in drug discovery and development processes to help international and Chinese pharmaceutical companies efficiently discover and develop drugs.
China Gateway Biologics provides biologics manufacturing services for the biopharmaceutical industry for Chinese and international customers.
ChemPartner is a contract research organisation serving the pharmaceutical and biotechnology industry. It provides integrated services across drug discovery and development, including discovery biologics, chemistry and biology, as well as pre-clinical development.