Weekly insulin analogues approaching approval could herald change
At the ongoing Diabetes UK conference, experts highlighted the potential benefits and concerns surrounding weekly insulin candidates.
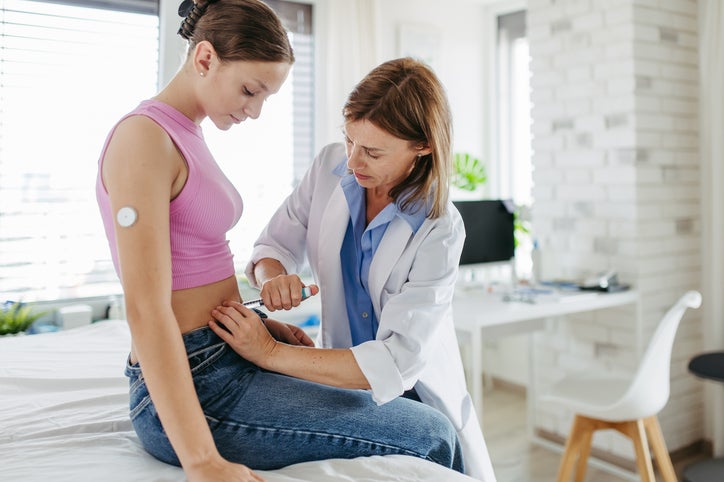
At the ongoing Diabetes UK conference, experts highlighted the potential benefits and concerns surrounding weekly insulin candidates.


For the process, the researchers employed a machine learning method called transfer learning.
The pharmaceutical industry continues to be a hotbed of patent innovation. Activity is driven by the evolution of treatment paradigms,...


Pharma Technology Focus is our digital magazine, free to read online on all devices. Click the magazine cover to read the latest issue. You can subscribe for free to have each new issue delivered to your inbox.
TetraScience and Google Cloud link for AI-driven drug developments
Bayer and Google Cloud to develop AI solutions for radiologists
Q&A: BARDA wants to back tech that can combat multiple infections, says director
Can pharma overcome generative AI’s bias problem?