HIV, AIDS and malaria are massive problems throughout Africa. The drugs to treat and prevent these diseases are readily available in the Western world but they are produced by large pharmaceutical companies and cost a great deal to import to Africa. Because these diseases are so widespread across Africa the cost of providing medication for everyone is prohibitive. The poorer African countries simply cannot afford these essential drugs.
Uganda is one of the success stories in the treatment of and public education about AIDS/HIV. The prevalence of the virus in the country has fallen from over 30% in the 1990s to around 6.44% inm 2008, which is encouraging, although some studies have put the prevalence at around 17%.
Since the 1990s India has produced antiretrovirals for the African market as the cheaper generic versions. However, a change in the patent laws in 2005, which came into force in January 2008 as a ratification of the Trade-Related Aspects of Intellectual Property Rights (TRIPS) provisions of the WTO, has prevented this. Uganda has now sought its own avenue of supply for these generics.
Own supply
Other African countries such as Egypt, Nigeria, South Africa and soon Mozambique have set up facilities to produce their own HIV drugs in generic versions. These countries have successfully provided for their own population as well as now exporting to other African countries in the same position.
Uganda also started to produce the required drugs in the cheaper generic versions. The two therapies being produced are antiretrovirals and first-line antimalarials.
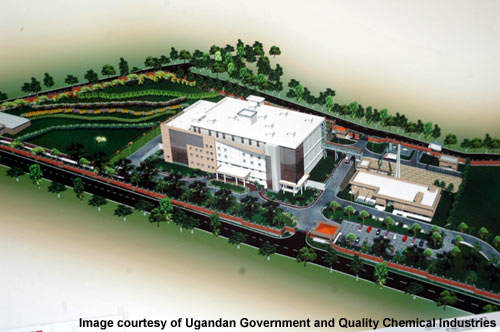
Quality Chemical Industries plant
In October 2007 a $38m pharmaceutical plant was set up in Uganda on a 15-acre site southwest of the capital Kampala, by the drug importer Quality Chemical Industries to produce antiretroviral drugs for the domestic market. The architects/engineers for the plant were ARNITA Engineers.
Quality Chemical Industries is in the process of completing registrations of its product in Kenya. The company plans to begin exports to neighbouring East African countries, including Burundi, Rwanda, Tanzania, the Democratic Republic of Congo and Sudan.
The Indian pharmaceutical company Cipla holds a 42% stake in Quality Chemical Industries. Cipla provided the technology and the expertise to get the plant set up; the plant now provides an outlet for Cipla to produce these important drugs for the African market. . In November 2009, TLG Capital acquired a 8.2% stake in the plant. Capitalworks Investment Partners of South Africa also owns an 8.2% stake.
The active pharmaceutical ingredients to produce the drugs are imported from India at a lower import cost – this was allowed under the new law.
The new facility was inaugurated by President Yoweri Museveni and the health minister Dr Stephen Malinga in January 2008. From October 2007 to January 2008 the plant was set up and validated.
In July 2009, the plant was cleared to produce AIDS and malaria drugs by an international committee of the Red Cross. Auditors from the committee conducted an inspection of the plant’s storage and manufacturing facilities. The auditors determined that the facilities are clean and acceptably designed according to World Health Organization recommended good manufacturing practices.
The plant was approved as a contract manufacturing site by the World Health Organization in June 2010. The inspection established the facility’s compliance with Good Manufacturing Practice.
Quality Chemical’s plant has to overcome several problems, including an intermittent power supply and trouble in sourcing artemesia (the raw material for producing ACT) before starting full production.
New Expansion
In February 2011, Quality Chemicals Industries stated it plans to invest $80m in a two-phased expansion. The $30m first phase of the project will expand the capacities at Quality Chemical Industries’ generic AIDS and malaria-drug plants in Kampala. The remaining $50m investment will be used to add a new production line for pharmaceutical ingredients during the second phase.
Funding for the project will be raised from the shareholders.
Production
The plant started producing the triple-therapy combination Triomune (containing lamivudine, stavudine and nevirapine) and the antimalarial therapy Lumartem (containing artemisinin and lumefantrin) following its inauguration. However, the plant only started production in February 2009.
The plant did not manufacture Triomune or Lumartem but produced ARV Duovir-N which is Uganda’s first-line treatment for AIDS. To fulfil its contracts for the drugs, Quality Chemical started importing Cipla’s ACT Lumartem.
The plant produces two million tablets per day per shift and 600 million tablets per year. The immediate future production target is 1.2 billion tablets after the expansion. The facility employs 500 personnel including 200 scientists for research and development.
Orders
AIDS and HIV are such a big problem in Uganda that the government has made provision in the budget to purchase medication for treatment. The first orders for antiretroviral therapies have already been received for the first quarter.
Some of the drugs will also be purchased by donor and charitable organisations for the Ugandan people.
Emmanuel Katongole is the MD of the new plant and has said that the drugs will be around 30% cheaper than the imported versions giving a monthly cost for the triple therapy of around $9.
It is hoped that the government will also help to fill gaps in the distribution/supply chain for the new drugs so that they get to where they are needed.
Qualifying distribution outlets must have a laboratory, a trained doctor and a counsellor so this currently only includes hospitals and medical centres. The Ugandan Government has been criticised for allowing over $1m worth of ARVs to expire before they could be distributed, due to a lack of outlets; their shelf life is only 18 to 24 months.