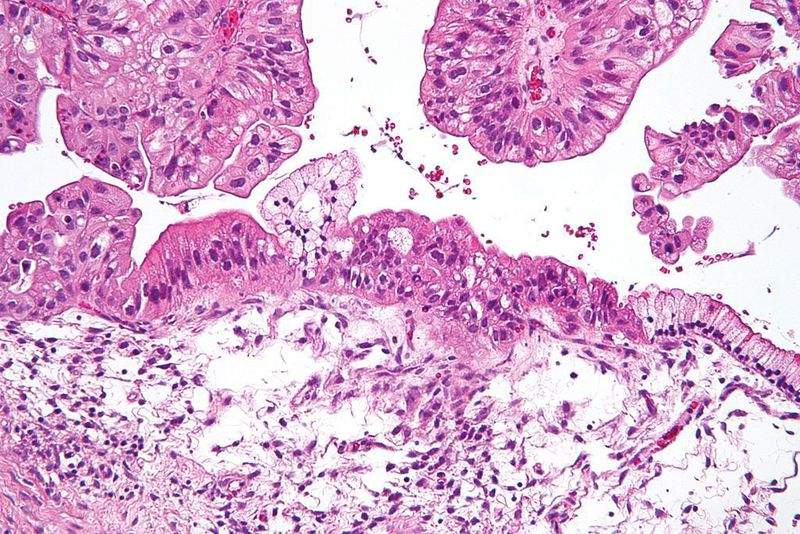
The US Food and Drug Application (FDA) has agreed for priority review of a supplemental New Drug Application (sNDA) of AstraZeneca’s Lynparza (olaparib) as a maintenance treatment for ovarian cancer.
The sNDA seeks approval for newly-diagnosed, BRCA-mutated (BRCAm) advanced ovarian cancer patients in complete or partial response after first-line standard platinum-based chemotherapy.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Being developed in alliance with Merck, Lynparza is a poly ADP-ribose polymerase (PARP) inhibitor designed as a targeted therapy for DNA damage response (DDR) pathway deficiencies, such as BRCA mutations.
The drug holds approvals in more than 60 countries for platinum-sensitive relapsed ovarian cancer, irrespective of the BRCA status. It is also approved in certain markets to treat germline BRCAm HER2-negative metastatic breast cancer.
Lynparza sNDA for BRCA-mutated advanced ovarian cancer includes results from the pivotal Phase III SOLO-1 clinical trial performed to assess the drug’s efficacy and safety as maintenance monotherapy.
The randomised, double-blinded, placebo-controlled, multi-centre trial enrolled a total of 391 participants with a deleterious or suspected deleterious BRCA1 or BRCA2 mutation.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAstraZeneca chief medical officer and Global Medicines Development executive vice-president Sean Bohen said: “The remarkable results of the SOLO-1 trial, which showed that 60% of women with newly diagnosed, advanced BRCA-mutated ovarian cancer remained progression-free at three years, highlight the potential of Lynparza as a maintenance therapy in the first-line setting.”
Primary endpoint of the trial was progression-free survival (PFS) and secondary endpoints included time to second disease progression or death, time to first subsequent treatment and overall survival.
Data revealed statistically-significant and clinically-meaningful improvement in PFS with Lynparza, compared to placebo. The drug is said to have minimised the risk of disease progression or death by 70%.
In the Lynparza arm, 60% of patients remained progression-free at 36 months, compared to 27% who received placebo.
Last month, the FDA granted orphan drug designation (ODD) for Lynparza (olaparib) to treat pancreatic cancer.
AstraZeneca and Merck partnered in July last year to co-develop and co-commercialise for a variety of cancer types.




