Aileron Therapeutics has filed a patent for aqueous pharmaceutical formulations that contain peptidomimetic macrocycles or their salts. These formulations can bind to MDM2 and/or MDMX proteins and may be used for treating diseases and disorders. GlobalData’s report on Aileron Therapeutics gives a 360-degree view of the company including its patenting strategy. Buy the report here.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
According to GlobalData’s company profile on Aileron Therapeutics, peptide pharmacophores was a key innovation area identified from patents. Aileron Therapeutics's grant share as of June 2023 was 1%. Grant share is based on the ratio of number of grants to total number of patents.
Pharmaceutical formulations for treating diseases using peptidomimetic macrocycles
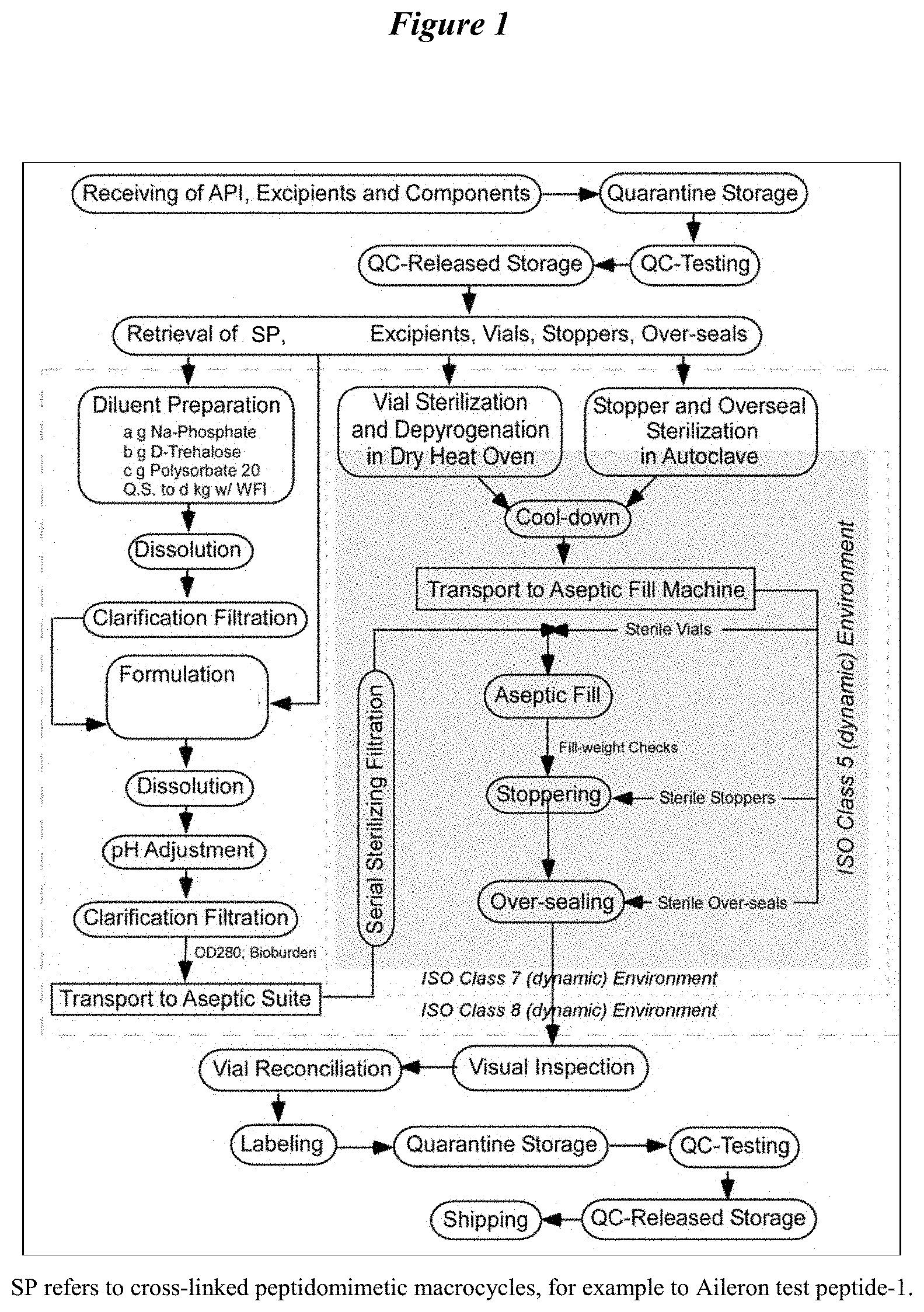
A recently filed patent (Publication Number: US20230127248A1) describes an aqueous pharmaceutical formulation in a unit dosage form. The formulation includes a peptidomimetic macrocycle or a pharmaceutically acceptable salt, a buffering agent, a stabilizing agent (polysorbate 20), and a tonicity agent (trehalose). The peptidomimetic macrocycle has a specific structure and length, with a range of 14 to 20 amino acids. It also has a von Heijne value of 2 to 9 and a percent alanine content of 15% to 40%. The C-terminal amino acid of the macrocycle is hydrophobic. The formulation may also include an a-helix and a pharmaceutically acceptable salt such as sodium, potassium, lithium, calcium, zinc, or magnesium salt.
The patent also describes specific characteristics of the aqueous pharmaceutical formulation. The total degradation products of the peptidomimetic macrocycle in the formulation should be less than 1.0% when stored at 40°C for one month. The osmolarity of the formulation should be between 250 and 1000 milliosmoles per kilogram. Additional ingredients such as glucose, fructose, galactose, sucrose, lactose, maltose, or a mixture thereof may be included. The pH of the formulation should be in the range of 6.0 to 8.0 or 4.0 to 9.0. The peptidomimetic macrocycle has a molecular weight between 1800 and 2000 D.
The patent also includes a method for making the aqueous pharmaceutical formulation. The method involves adding more than 15 mg/mL of the peptidomimetic macrocycle or its salt to water or an aqueous solution. The formulation should contain less than 2% w/v of any micelle forming agent. The peptidomimetic macrocycle used in the method is capable of binding to the MDM2 and/or MDMX proteins. The method may also include adding a sodium salt of the macrocycle, adjusting the pH of the solution, and filtration of the final formulation.
Overall, this patent describes a specific aqueous pharmaceutical formulation containing a peptidomimetic macrocycle with defined characteristics. The formulation aims to provide stability and suitable properties for administration to a subject without dilution.
To know more about GlobalData’s detailed insights on Aileron Therapeutics, buy the report here.
Data Insights
From

The gold standard of business intelligence.
Blending expert knowledge with cutting-edge technology, GlobalData’s unrivalled proprietary data will enable you to decode what’s happening in your market. You can make better informed decisions and gain a future-proof advantage over your competitors.



