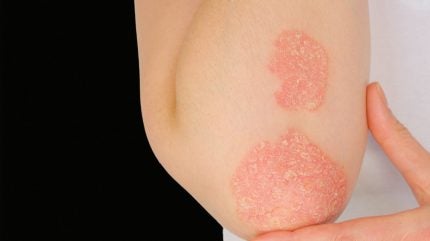
Johnson & Johnson (J&J) has submitted a new drug application (NDA) seeking approval from the US Food and Drug Administration (FDA) for its oral peptide, icotrokinra, to treat moderate to severe plaque psoriasis (PsO) in adults and children aged 12 years and above.
Icotrokinra was co-discovered and is currently being developed through a licensing and collaboration agreement between Protagonist and J&J.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
J&J holds exclusive worldwide rights to advance icotrokinra through Phase II clinical trials and beyond, as well as to commercialise any compounds resulting from the research for a wide array of indications.
Icotrokinra selectively blocks the Interleukin-23 (IL-23) receptor, which plays a critical role in inflammatory responses associated with PsO.
The NDA submission includes data from four pivotal Phase III studies within the ICONIC clinical development programme: ICONIC-LEAD, ICONIC-TOTAL, ICONIC-ADVANCE 1 and ICONIC-ADVANCE 2.
Johnson & Johnson innovative medicine immunodermatology and respiratory disease area lead and vice-president Liza O’Dowd stated: “The rapid patient enrolment across our ICONIC clinical programme underscores the unmet need for an advanced plaque psoriasis treatment that meaningfully addresses their needs and preferences.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“Given the breadth and depth of our studies, along with the robust clinical results reported to date, we are confident that icotrokinra has the potential to transform how physicians and patients think about plaque psoriasis care, establishing a new standard in the treatment of this immune-mediated disease.”
PsO is an immune-mediated disease characterised by rapid skin cell proliferation leading to inflamed plaques that can cause discomfort or pain.
In July 2025, J&J submitted a request to the European Medicines Agency (EMA) to expand the indications for Akeega (a combination of niraparib and abiraterone acetate) in the treatment of prostate cancer.
The company aims to include a hormone-sensitive indication for the therapy, which received its initial approval in 2023.




