
Japanese companies Eisai and Meiji Seika Pharma have signed an agreement for the commercialisation of safinamide to treat Parkinson’s disease in Japan and Asia.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Caused by degeneration of the dopamine nervous system, Parkinson's disease leads to motor impairment, including shaking in the limbs, muscular rigidity and brachybasia.
Safinamide is a selective monoamine oxidase B (MAO-B) inhibitor, which reduces the degradation of excreted dopamine and helps to maintain the density of dopamine in the brain.
It also blocks sodium ion channels and inhibits glutamate release, and has potential as a new Parkinson’s disease treatment that possesses dopaminergic, as well as non-dopaminergic mechanisms.
Under the agreement, Eisai will acquire exclusive rights to safinamide to market in Japan and also to develop and market in seven Asian countries.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataSafinamide is under clinical development by Meiji in Japan and the trials will continue, following which a manufacturing and marketing authorisation application would be submitted.
Meanwhile, Eisai will conduct clinical trials to obtain regulatory approval and submit the applications in Asia.
Safinamide will be manufactured and supplied to Eisai by Meiji for Japan and Asia.
Under the agreement, Meiji will also receive an upfront payment from Eisai, as well as developmental milestone and sales royalty payments.
Safinamide was discovered and developed by Italy’s Newron Pharmaceuticals, which signed a licensing agreement with Meiji in 2011.
The agreement granted exclusive rights to Meiji to develop, manufacture and commercialise the drug in Japan and Asia.
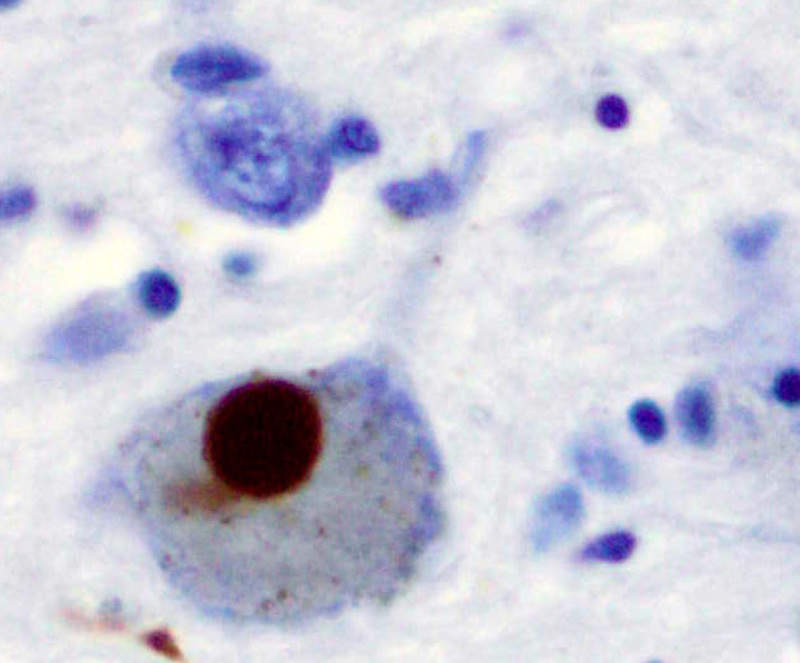
Image: Immunohistochemistry for alpha-synuclein showing positive staining (brown) of an intraneural Lewy-body in the Substantia nigra in Parkinson's disease. Photo: courtesy of Marvin 101.




