
Shire has filed marketing authorisation application (MAA) for lifitegrast as a treatment for patients with dry eye disease in Europe.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Dry eye is a multifactorial disease of the ocular surface that is often chronic and can also be progressive.
It is commonly associated with eye dryness and overall eye discomfort, accompanied by stinging and burning of the eye, as well as fluctuating blurry vision.
Lifitegrast is a lymphocyte function-associated antigen-1 (LFA-1) antagonist.
The treatment binds to the integrin LFA-1, a cell surface protein found on leukocytes and blocks the interaction of LFA-1 with its cognate ligand intercellular adhesion molecule-1 (ICAM-1), which plays a major role in ocular surface inflammation.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIf approved, the dry-eye disease therapy would be the first and only treatment in a new class of drugs (LFA-1 antagonist) to address the signs and symptoms of dry eye disease in European adults.
Shire Research and Development Clinical Development head Howard Mayer said: “This submission is another important milestone for lifitegrast and the millions of patients living with dry eye disease, which can impact a person’s vision-related quality of life, affecting daily activities such as reading and using computers.
“Shire is committed to continued innovation in ophthalmics, where there are opportunities to address unmet need and improve the lives of patients.”
The MAA submitted for lifitegrast is supported by the largest development programme for an investigational-stage dry eye disease candidate, comprising five clinical trials with more than 2,500 patients.
During the studies, the signs of dry eye disease were measured using corneal staining and the symptoms by using patient reported eye dryness score (EDS).
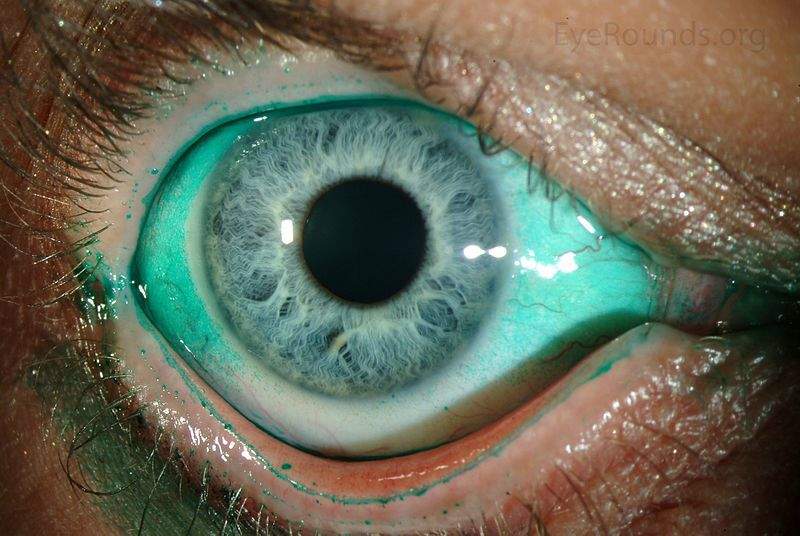
Image: Diffuse lissamine green staining in a person with severe keratoconjunctivitis sicca. Photo: courtesy of Jesse Vislisel, MD and Brice Critser, CRA.


