
Takeda Pharmaceutical has filed a new drug application (NDA) for vedolizumab with Japan’s Ministry of Health, Labour and Welfare to treat adult patients with moderately to severely active ulcerative colitis (UC).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
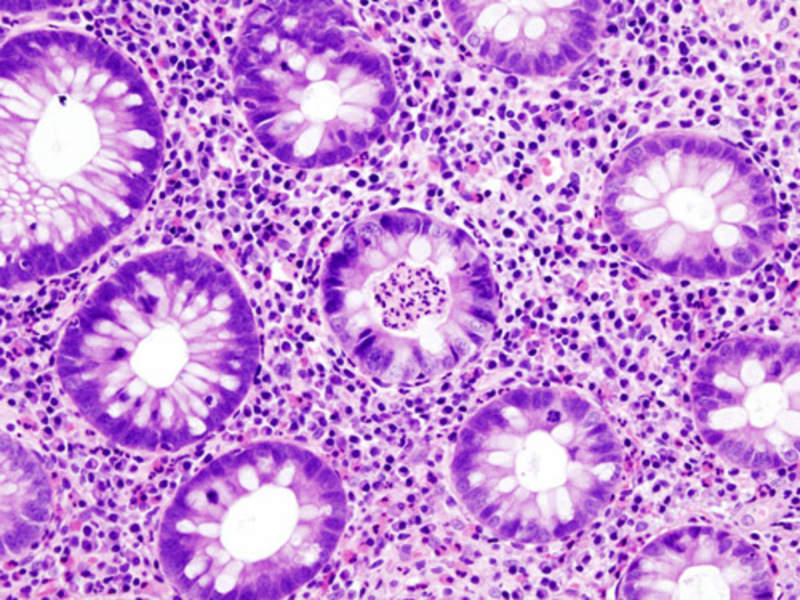
UC is one of the most common forms of inflammatory bowel disease (IBD), as well as a chronic, relapsing, remitting, inflammatory condition of the gastrointestinal (GI) tract that is often progressive in nature.
Vedolizumab is an investigational humanised monoclonal antibody that has been approved for adults with moderate-to-severe UC or Crohn's disease (CD) in more than 60 countries worldwide.
People suffering from UC have an increased number of inflammatory white blood cells entering the mucosal lining of the bowel.
The investigational treatment has been developed to reduce the inflammation by blocking the movement of the white blood cells into the inflamed gut tissue.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataTakeda Development Centre Japan head Toshiro Heya said: “UC is a chronic, progressive inflammatory disease of the large intestine, which typically affects young adults.
“The disease has a high impact on their quality of life with symptoms, including diarrhoea, rectal bleeding, incontinence and abdominal pain.
“Through this submission, we may provide an alternative treatment option to the increasing number of people with UC in Japan.”
The NDA submission is based on the data obtained from a multicentre, randomised, double-blind, placebo-controlled, parallel-group Phase III trial, Study CCT-101.
The trial involves 292 moderate or severe UC patients from Japan and investigates the efficacy, safety and pharmacokinetics of vedolizumab induction and maintenance treatment.
The filing also included data from the international, randomised, double blind, placebo-controlled GEMINI I pivotal Phase III study of vedolizumab induction and maintenance treatment assessing 895 patients with moderate-to-severe UC.
Image: Histopathological image of the active stage of ulcerative colitis. Photo: courtesy of User: KGH.




