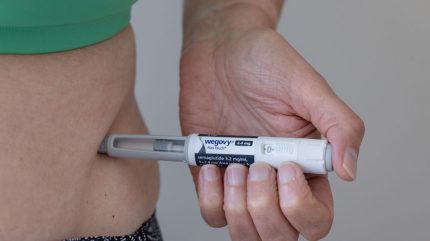
Danish big pharma Novo Nordisk has been given the go-ahead for its star weight loss drug, Wegovy (semaglutide), in patients with metabolic dysfunction-associated steatohepatitis (MASH) who have moderate-to-advanced liver scarring.
This makes it the first glucagon-like peptide 1 receptor agonist (GLP-1RA) to be approved by the US Food and Drug Administration (FDA) for this indication.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Wegovy obtained accelerated approval in MASH based on the outcome of the Phase III ESSENCE trial (NCT04822181), which found that a weekly dose of subcutaneous Wegovy could resolve steatohepatitis – a severe form of fatty liver disease – in 63% of patients. This was compared to 34% in the placebo group.
Wegovy also helped to improve liver fibrosis in 37% of patients compared with 22% in the placebo group.
The drug’s full approval is conditional on the results of the ongoing ESSENCE study, which will continue to assess the long-term benefits of Wegovy alongside a reduced-calorie diet and increased physical exercise.
Novo Nordisk’s best-selling weight loss drug now joins Madrigal Pharmaceuticals’ Rezdiffra (resmetirom) on the market, which was the first medication to be approved in noncirrhotic MASH in March 2024.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAccording to analysts at Pharmaceutical Technology’s parent company, GlobalData, Rezdiffra is expected to reach blockbuster status by 2026, raking in $1.1bn for Madrigal after two years on the market. By 2031, the drug is forecast to pull in $4.8bn for Madrigal – highlighting its rapid growth trajectory moving forward.
It will be interesting to see how Wegovy competes with Rezdiffra in this indication, as the thyroid hormone receptor beta (THR-β) agonist’s fibrosis improvements were similar to those seen with Wegovy. However, it does have the benefit of oral administration, which is more convenient for patients.
GlobalData pharma analyst Jay Patel said: “This outcome makes Wegovy the second US-approved asset in MASH, applying competitive pressure to Madrigal’s Rezdiffra.
“Nevertheless, Rezdiffra’s liver-directed effect may have advantages in treating advanced fibrosis, and Madrigal may choose to emphasise this in future marketing, although fibrosis improvement results from the Phase III MAESTRO-NASH trial of Rezdiffra were broadly similar to ESSENCE.”
However, the drug’s approval will still be welcome news for Novo Nordisk, which is currently in a head-to-head battle with Eli Lilly for GLP-1RA glory.
The two companies are currently fighting for market share in the type 2 diabetes (T2D) and weight loss markets, with sales of Nordisk’s Ozempic and Wegovy slowing due to competition from Lilly’s Zepbound and Mounjaro (tirzepatide), which brought in $8.57bn in Q2.
However, Lilly’s oral GLP-1RA candidate, orforglipron, failed to surpass the efficacy of Nordisk’s oral semaglutide in a Phase III trial in weight loss, causing the former’s shares to drop by 12% to $656.19 after market open post-data release.
Though Novo Nordisk attained approval in MASH first, Lilly is also investigating Zepbound in this indication, with a Phase IIb trial having shown signs of efficacy.




