Danish pharmaceutical company Novo Nordisk broke ground on its diabetes active pharmaceutical ingredients (DAPIs) production facility in Clayton, North Carolina, in March 2016.
The ground-breaking ceremony was attended by North Carolina Governor Pat McCory and Novo Nordisk president Lars Rebien Sorensen, as well as community members, employees and policymakers.
The company is investing around $1.85bn to develop the production facility, which is expected to be fully operational by 2020.
The project is expected to receive around $94m in tax rebates from Johnston County over a period of 15 years.
This investment is part of the company’s strategy to invest around $2bn in its production facilities in Clayton, as well as in Malov and Kalundborg, Denmark.
Details of Novo Nordisk’s DAPI production facility in North Carolina
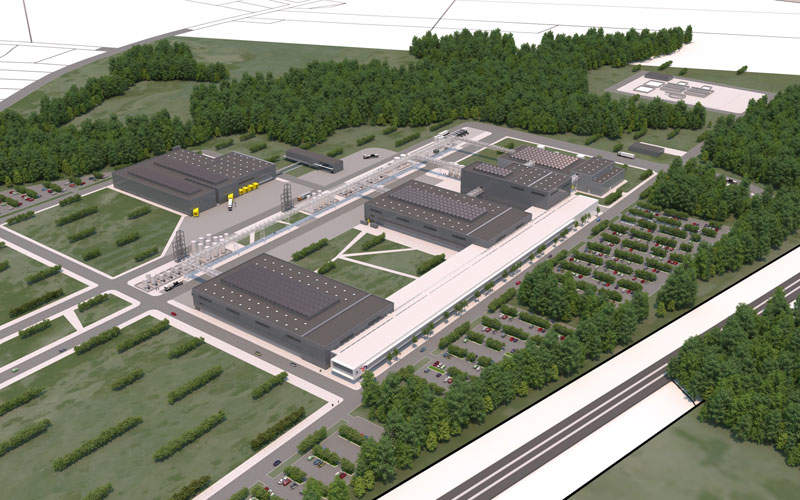
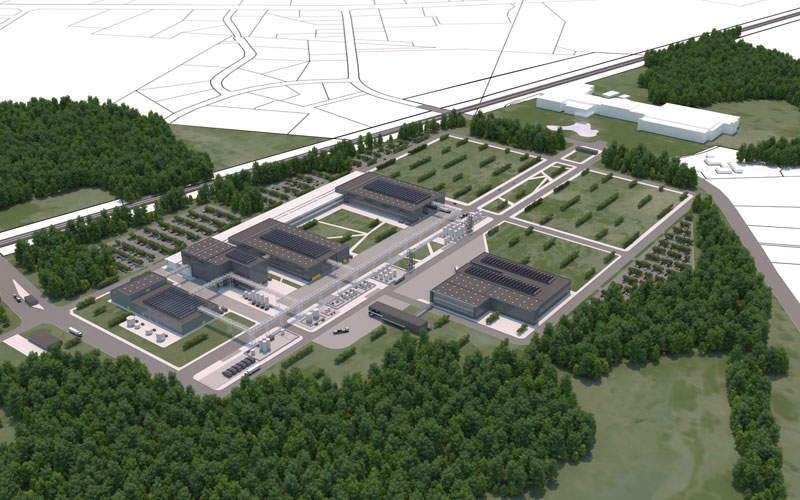
The DAPI manufacturing plant will occupy an 833,000ft² area and will have a total footprint of 147,639ft².
It is being built next to Novo Nordisk’s existing 452,084ft² (42,000m²) facility, which is involved in the formulation, filling and packaging of diabetes medicines. The existing plant also assembles and packages Flex Pen and Flex Touch prefilled insulin devices for the US market.
The new plant will produce active pharmaceutical ingredients (APIs) for a range of current and future GLP-1 and insulin products for Novo Nordisk.
Once the plant becomes fully operational, the DAPI production facility in Clayton will be named DAPI-US (Diabetes Active Pharmaceutical Ingredients-US) and will form part of the company’s DAPI production facility in Kalundborg, which will be named DAPI-Denmark.
Benefits of the DAPI production plant in North Carolina
The site will enlarge Novo Nordisk’s production capacity for diabetes care products and play an important role in helping the company meet future demand for diabetes medicines in the US. The expansion will also reinforce Novo Nordisk in its efforts to continue as a leader in diabetes care worldwide.
The project is expected to generate around 2,500 jobs during its construction phase and 700 permanent jobs. Employees will be offered an average salary of $68,000, which is higher than the current average salary. The existing facility in Clayton currently employs more than 700 people.
Marketing commentary on Novo Nordisk
Novo Nordisk is a global healthcare organisation based in Denmark, with 90 years of innovation and leadership in diabetes care.
As well as diabetes, the company has experience in developing treatments for other serious chronic conditions such as haemophilia, growth disorder and obesity.
Novo Nordisk manufactures products in five areas, namely diabetes care, haemophilia, growth hormone therapy, obesity and hormone replacement therapy.
The company currently employs around 41,000 people across 75 countries and markets its products in more than 180 countries.