No Filter Selected
No Filter Selected
No Filter Selected
No Filter Selected

Vertex Pharmaceuticals' Boston Campus
Massachusetts-based biotechnology company Vertex Pharmaceuticals (Vertex) is currently relocating its headquarters from Cambridge to Fan Pier, on the Boston Waterfront....
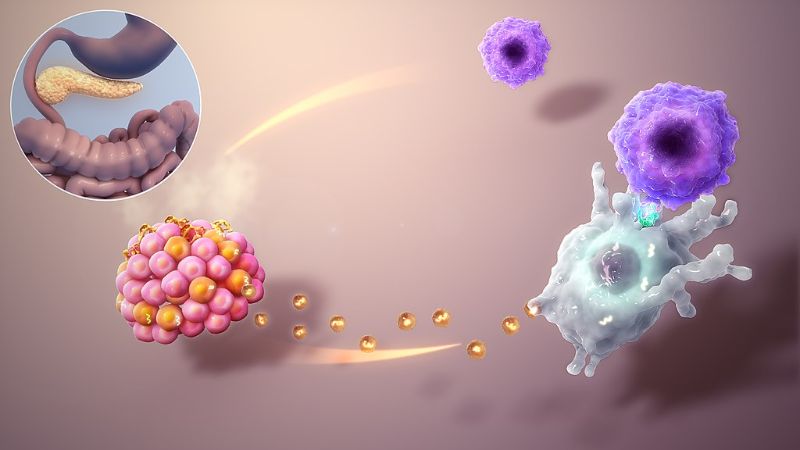
Vertex to buy Semma Therapeutics for $950m
Vertex Pharmaceuticals will acquire all outstanding shares of biotechnology firm Semma Therapeutics for a cash sum of $950m.

Vertex to acquire Exonics Therapeutics for $245m
Vertex Pharmaceuticals has signed a definitive agreement to acquire all outstanding shares of gene editing therapies developer Exonics Therapeutics for an upfront payment of $245m.

Vertex’s cystic fibrosis drugs become publicly available in Denmark
Vertex Pharmaceuticals has signed an access agreement with Denmark’s pharmaceutical procurement agency Amgros for public hospital use of all current and future drugs for mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene.
Health Canada approves Vertex hepatitis drug
Vertex Pharmaceuticals has received Health Canada approval for Incivek (telaprevir) tablets for a broad group of people with genotype 1 chronic hepatitis C with compensated liver disease, including cirrhosis. Incivek is approved for use in all three major groups of people who have not a
EC approves Vertex’s Symkevi for cystic fibrosis
The European Commission has approved London-based Vertex’s Symkevi (ivacaftor+tezacaftor) in combination with ivacaftor for patients with specific mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene.

Vertex and Kymera to identify protein degradation therapeutics
Vertex Pharmaceuticals and Kymera Therapeutics have formed a strategic research and development (R&D) alliance to develop protein degradation medicines for various targets.

Vertex signs agreement to acquire ViaCyte for $320m
Through the acquisition, Vertex plans to advance its potentially curative VX-880 programmes in T1D.

Vertex and Entrada partner for EEV therapeutics development
Entrada is entitled to get potential research, development, regulatory, and commercial milestone payments, worth up to $485m.
Suzetrigine by Vertex Pharmaceuticals for Radiculopathy: Likelihood of Approval
Suzetrigine is under clinical development by Vertex Pharmaceuticals and currently in Phase II for Radiculopathy. According to GlobalData, Phase II...
Vertex and NHS England agree reimbursement for Alyftrek
Alyftrek is approved for individuals aged six years and older with at least one F508del mutation.

NHS England calls Vertex an ‘extreme outlier’ in conduct of Orkambi negotiations
During a House of Commons Health and Social Care Committee witness session on the availability of Vertex’s Orkambi and other cystic fibrosis (CF) drugs on the National Health Service (NHS), representatives from NHS England and the UK’s pricing regulator National Institute for Health and Care Excellence (NICE) called Vertex an ‘extreme outlier’ in terms of its engagement with the UK’s healthcare authorities and their appraisal processes.

Vertex to use ImmunoGen technology to discover conditioning agents
Exclusive rights to the technology will remain with ImmunoGen for all targets not covered by the Vertex licence.

Vertex and Obsidian enter agreement to develop gene-editing treatments
Vertex Pharmaceuticals (VRTX) and Obsidian Therapeutics have entered a strategic research partnership and licensing agreement to discover and develop novel therapies that regulate gene editing for treating serious diseases.
Vertex-Semma $950m acquisition: bringing diabetes into the pipeline
Vertex Pharmaceutics has announced it has entered into a definitive agreement to acquire Semma Therapeutics for $950m. Semma is an early-stage biotech that is developing stem cell therapies for type 1 diabetes patients; the deal with introduce a new disease area into Vertex’s pipeline.

Vertex-Affinia partnership: expanding the gene therapy toolkit
Building on the success of its gene-targeted cystic fibrosis therapies, Vertex has begun to expand into new disease areas that would benefit from a similar gene therapy approach. To this end, it has begun to develop a toolkit of technology to support drug development in new spaces, including DMD and DM1, bringing on-board AAV capsid-focused Affinia.
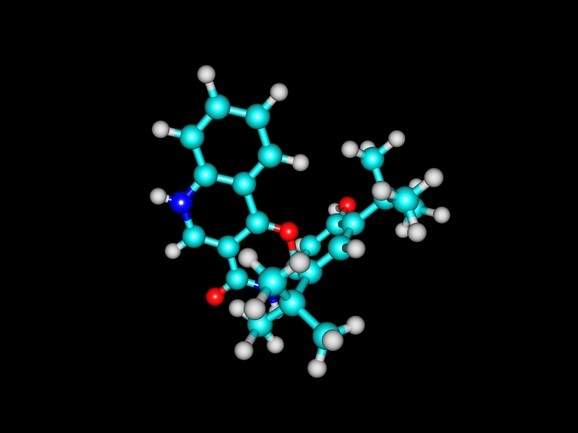
NICE suspends review of Vertex’s Symkevi for cystic fibrosis
The UK’s National Institute for Health and Care Excellence (NICE) has suspended its review of Vertex Pharmaceuticals’ cystic fibrosis (CF) drug Symkevi (tezacaftor/ivacaftor) after the company failed to submit evidence to NICE regarding the clinical and cost-effectiveness of the therapy.

Vertex shares up after full-year product revenue guidance boost
Vertex is approaching two key PDUFA dates in January for new treatments in cystic fibrosis and pain respectively.

Vertex and NHS England reach impasse over cystic fibrosis drug price
Cystic fibrosis (CF)-focused Vertex has declared that its recent negotiations with NHS England regarding the price of its drug Orkambi (ivacaftor+lumacaftor) for CF have been inconclusive.

Scotland reaches access agreement with Vertex for CF drug Orkambi
Vertex Pharmaceuticals has announced two of its cystic fibrosis (CF) drugs, Orkambi (lumacaftor-ivacaftor) and Symkevi (tezacaftor/ivacaftor) will now be available to eligible patients in Scotland through the National Health Service (NHS).
