
Cell and gene therapies represent a groundbreaking advancement in medical science, offering transformative opportunities for treating complex and previously untreatable diseases. These therapies function by directly modifying genetic material or utilizing whole cells to repair, replace, or regenerate damaged tissues and genes at the cellular level, thereby targeting the fundamental causes of diseases rather than merely alleviating symptoms.
Initially, the most significant applications of cell and gene therapies emerged within oncology, particularly blood cancers, where CAR-T therapies have demonstrated unprecedented success in treating previously resistant or recurrent malignancies. However, the scope of advanced therapies is rapidly expanding beyond oncology. There is growing evidence and clinical development in fields such as autoimmune diseases, neurological conditions, cardiovascular diseases, and rare genetic disorders. Each of these therapeutic domains represents substantial areas of unmet medical need, where traditional pharmaceutical interventions have historically been inadequate or limited in effectiveness.
As research progresses and regulatory pathways mature, the potential for advanced therapies to revolutionize healthcare continues to increase dramatically. According to GlobalData analysis, the market for CGTs, worth just under $6 billion in 2023, will grow at a massive 43% compound annual growth rate (CAGR) in the runup to 2030. The broadening spectrum of indications for these therapies reflects not only scientific progress but also the increasing understanding and management of the unique logistical, regulatory, and operational challenges these personalized treatments present. AI is already transforming target identification and drug development and has the potential to significantly improve scalability.
However, supply chain disruptions are impacting all therapeutic areas, and, if not resolved effectively, could impact treatment availability and the launch of new products.
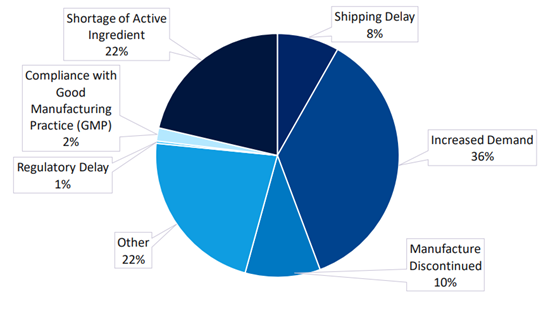
In a recent survey by GlobalData[i], “supply chain challenges” was listed as one of the top industry trends expected to have ’the greatest impact on the pharma sector in the next 12 months.’ It offers an insight into how increasingly tailored therapy supply chains make delivering products to specific patients challenging. And the financial burden associated with these therapies is substantial. The high costs stem from the need for specialized facilities, utilities and cycle management, all of which are essential for ensuring the quality and efficacy of these treatments.
Cell- & gene-therapy supply chains look deceptively short (“vein-to-vein”), yet every dose is a micro-batch made to one patient’s specification. As soon as the first clinical successes arrive, demand rises sharply, and three structural frictions appear:
- Manual, ad-hoc coordination across dozens of actors:
- Spreadsheets, chat groups and phone calls connect treatment-center nurses, planners, CMOs, couriers, and QA reviewers. Response times and data accuracy do not keep pace with order volume.
- Finite, clinic-bound capacity
- Slotting apheresis, vector manufacture, cell processing and release tests must be synchronized, if one slips the whole schedule ripples.
- Limited treatment-center network
- Without standardized digital workflows each new center triggers fresh integration work, slowing commercial ramp-up and delaying patient access.
First-generation CGT developers have built a lot of point-solutions, that grew over time, addressing the specific needs as they arise with custom-built solutions. The consequence is that, often times, CGT IT landscapes are complex, costly, slow, over-specific, and not scalable, leaving companies stuck in digital silos rather than the connected, predictive plants needed for volume growth
Where can pharma industry professionals, tasked with architecting a modern approach to scaling a CGT product, turn for salvation?
A proper digital platform becomes the inflection point. Instead of people phoning each other to chase shipments or update dates, every step emits an event; downstream systems subscribe, react and update plans automatically. That shift:
- Removes latency and data re-entry errors.
- Lets planners simulate scenarios and commit only when plant-level capacity is truly available.
- Standardizes the “last mile” process at each treatment center so new sites can go live in days, not months.
There is also one “new kid on the block”: According to those surveyed for GlobalData’s 2024 Digital Transformation and Emerging Technology in the Healthcare Industry survey, 20% of them stated that they believed that AI is going to be highly beneficial for improving the pharma supply chain and distribution. A new generation of pharmaceutical orchestration platforms is already putting it into practice.
AI enhanced supply chains
AI is revolutionizing the pharmaceutical supply chain by automating communication and data sharing, facilitating stakeholder integration, reducing delay, and improving control over suppliers and inventory management. It can also optimize manufacturing processes, reducing time for small batches and forecasting demand. Predictive analytics can identify potential issues before they occur, allowing companies to proactively address risks. AI can also help mitigate shipping delays by optimizing supply chain logistics and automating communication with suppliers, ensuring timely updates on inventory levels and shipping schedules.
AI offers a powerful suite of tools that can enhance efficiency, scalability and risk management within the pharmaceutical industry. By investing in AI technologies, companies can improve their ability to integrate multiple stakeholders, respond to time-sensitive demands and maintain compliance with industry regulations.
Cell and Gene Therapy Orchestration
Now, AI is making headway in some of the industry’s leading orchestration platforms. SAP Cell and Gene Therapy Orchestration , for example, is a solution that helps life sciences companies organize, plan and execute personalized cell and gene therapy treatments. It is “a treatment management platform and exception-management system” built to tackle the logistic, regulatory and process-orchestration challenges unique to CGT supply chains.
Key features include tracking patient and product details, performing digital order management, managing shipment order lifecycle, allowing confirmation of schedules and manufacturing slots and enabling digital document exchanges. Benefits include reduced data integration costs, reduced risk of quality noncompliance and reduced order fulfillment lead time[KA3] .Its robust product roadmap and guarantee of continuous investment helps futureproof users where other solutions may fall short. SAP CGTO also features an event-based architecture that enables seamless integration with any ERP system. It includes out-of-the-box connectivity with SAP S/4HANA. One leading firm has successfully implemented SAP CGTO integrated with S/4HANA, cutting integration lead times from half an hour to under 1 minute using SAP Integration Suite. Improvements in speed, reliability, and operational efficiency have been significant.
AI functionality is critical to SAP’s offering. The AI-enabled Control Tower for Advanced Therapies aims to improve patient access to personalized therapies by anticipating supply chain bottlenecks and suggesting alternative options to mitigate issues and exceptions. The system uses historical data to identify and propose solutions based on factors such as region, therapy, patient, treatment center and logistics.
SAP Supply Chain Control Tower guides users in the next steps to order fulfillment using core functionalities of SAP Cell and Gene Therapy Orchestration . The Control Tower is an AI engine that compares data to plan to identify deviations or delays that could jeopardize milestones. It compares plant milestones, plan times and phrasing exceptions if something is wrong.
The business impact of this solution is immense, as it increases manufacturing capacity and patient access, reduces effort in exception resolution and optimizes manufacturing capacity/patient access at scale. This solution is particularly beneficial for the pharmaceutical and biotech industry in the advanced therapies space, particularly for companies scaling with approved therapies or in late-stage clinical trials.
The solution includes AI-supported orchestration and exception management to maximize patient access to advanced therapies, proposing alternative manufacturing sites, transportation lanes, lead times and treatment schedules. It has the potential to have a significant impact on revenue generation for pharma companies in the advanced therapies space.
Strengthening collaboration – the CDMO angle
Contract Development and Manufacturing Organizations (CDMOs) play a pivotal role in today’s pharmaceutical supply chains. In the growing CGT space, their role is vital. As sponsors increasingly outsource manufacturing processes, synchronizing with CDMOs has become essential for maintaining compliance, accelerating turnaround and ensuring the integrity of sensitive therapies. AI-powered supply chain platforms – like SAP Business Network and Control Tower – can help bridge the data and visibility gaps that may exist between sponsors and CDMOs. These solutions enable seamless exchange of critical data points such as chain of identity and custody, manufacturing status, and raw material availability. And they can do this all while reducing reliance on manual interventions and legacy interfaces.
Traditional data exchanges between pharma firms and CDMOs often rely on flat files or PDFs, limiting visibility into real-time shop floor operations. For high-value therapies with short manufacturing cycles, this lack of transparency introduces costly inefficiencies – meaning the potential from AI-driven digital manufacturing tools and orchestration platforms cannot be ignored. These tools allow CDMOs to share operational insights directly from the manufacturing floor, empowering sponsors to monitor progress, manage working capital more effectively, and proactively resolve delays – all while guaranteeing compliance adherence by recording and sharing COI/COC events. As CGT supply chains evolve, integrating AI to support collaboration with CDMOs will be critical for reducing costs and scaling personalized therapies confidently.
AI interactive support
In addition to their functionality, AI solutions are making discovery and implementation of supply chain solutions more accessible than ever thanks to their natural language capabilities. SAP Joule, for example, is an AI enabled interactive support solution, designed to provide supply chain coordinators with a comprehensive, intuitive and accurate overview of critical treatment order information within the SAP Cell and Gene Therapy Orchestration platform. This interactive approach streamlines the review and approval process, enhancing operational efficiency and ensuring timely patient care. The solution empowers coordinators by allowing a unified view of all critical information, enabling them to make informed decisions more efficiently, reducing time and efforts required to gather and review data across multiple systems.
Supply chain coordinators need to review information from various sources, scattered across different applications and screens. This not only increases the time and effort required but often requires other tools to collate all the information correctly. However, Joule can bring together all the pertinent information about the order from a simple prompt, including shipment details with materials, processing activity information and associated ERP objects. Other prompts can provide pending items for action, status and patient eligibility checks. The system can even retrieve patient information from different sources and allow Patient Operations to review them.
The SAP Joule solution provides a comprehensive, intuitive and accurate overview of critical treatment order information, streamlining the review and approval process, enhancing operational efficiency and ensuring timely patient care. By leveraging AI-enabled Orchestration Insights, the solution empowers coordinators to make informed decisions more efficiently, reducing time and efforts required to gather and review data across multiple systems.
Intelligent Clinical Supply Management
For clinical trial organizers, SAP Intelligent Clinical Supply Management solution is perfect for implementing AI across their pipelines. This comprehensive solution integrates AI, machine learning and real-time analytics to optimize supply chains, forecast demand more accurately, streamline logistics and reduce supply cycle times. SAP Intelligent Clinical Supply Management integrates with CTMS and IRT to meet blinding and randomization needs, facilitate demand forecasting, manufacturing, packaging, labeling and shipments of clinical trial material.
Both large and mid-size companies already using SAP Intelligent Clinical Supply Management say that, by using the solution, they have enhanced visibility across clinical supply chains, minimized wastage and ensured timely drug delivery. One firm increased supply chain efficiency by 30% and reduced compliance issues by 50% by adopting SAP’s AI-driven cloud ERP solutions.
To learn more about SAP and Cell and Gene Therapy Orchestration solutions, download the free paper below.
[i] Survey fielded November 15, 2024, to December 4, 2024, and reported in Globaldata: The State of the Biopharmaceutical Industry, 2025 Edition.


