Y. Hartmanna, M. Dabrosb, R. Pierrec, C. Rhêmec, X. Gaoc
aHES de Fribourg, Bd de Pérolles 80, 1700 Fribourg, Switzerland, yves.hartmann@hefr.ch
bHES de Fribourg, Bd de Pérolles 80, 1700 Fribourg, Switzerland, michal.dabros@hefr.ch
cFrewitt SA, Rte du Coteau 7, 1763 Granges-Paccot, Switzerland, x.gao@frewitt.com
Introduction
Lactobacillus plantarum is a widely recognized probiotic microorganism, valued for its gut health benefits and immune-modulating properties. Industrial processes for formulating probiotics must ensure optimal delivery to the gastrointestinal tract, requiring uniform particle size and high viability. High-energy milling, such as with the PM-160 grinder from Frewitt SA (Granges-Paccot, Switzerland), offers many advantages in achieving desired particle size distribution (PSD) but poses challenges in preserving microbial viability. This study focuses on evaluating the PM-160’s capabilities, emphasizing the impact of grinding parameters on Lactobacillus plantarum viability and PSD.To analyze and compare the results objectively, statistical tools such as Student’s t-test were performed.1
Flexmill-Lab PM-160
This equipment (shown in Figure 1) is a high-performance R&D and small-scale production pin mill, capable of attaining a D(90) under 6 µm and a throughput of 40 kg/h. Grinding rotor speed ranges from 10’000 to 21’000 RPM (83 m/s to 175 m/s). The temperature of the ground product leaving the mill can be adjusted from -20 °C to 60 °C, enabling a wide range of applications.3
Method
Experimental Setup
The PM-160 was used under nitrogen atmosphere to reduce oxidative stress during grinding. Grinding speeds of 83, 101, 126, 151, and 175 m/s were tested with a constant feed rate of 10 rotations per minute which corresponds to approximately 1.5 grams of lyophilized bacteria per seconds. Lyophilized L. plantarum samples were introduced into the grinding chamber, and exit temperatures were recorded.

Biological Activity Assessment
Biological activity was measured using colony-forming unit (CFU) assays. Rehydrated bacterial samples were diluted and cultured on nutrient-rich agar plates, incubated at 37°C for 48 hours before CFU counting. Triplicates of every culture were done, and each measurement was repeated at least 6 times. Statistical significance between groups was evaluated using Student’s T-test (described below).
Particle Size Analysis
The Malvern Mastersizer 3000 was used to measure PSD for ground samples, focusing on D10, D50, and D90 values. Scanning electron microscopy (SEM) was used to validate these measurements and examine morphological changes in the samples.
Student’s T-test as statistical tool
Student’s T-test is a statistical method used to determine if there is a statistically significant difference between two sets of data. By performing the T-test on two sets of data, the P-value, which is the probability that the differences between those sets to be due to chance. Conventionally, a T-test with a P-value inferior to 0.05 is considered to be statistically significant, in other words that the differences between the two sets of data is unlikely to be due to chance.2
Particle aspect assessment
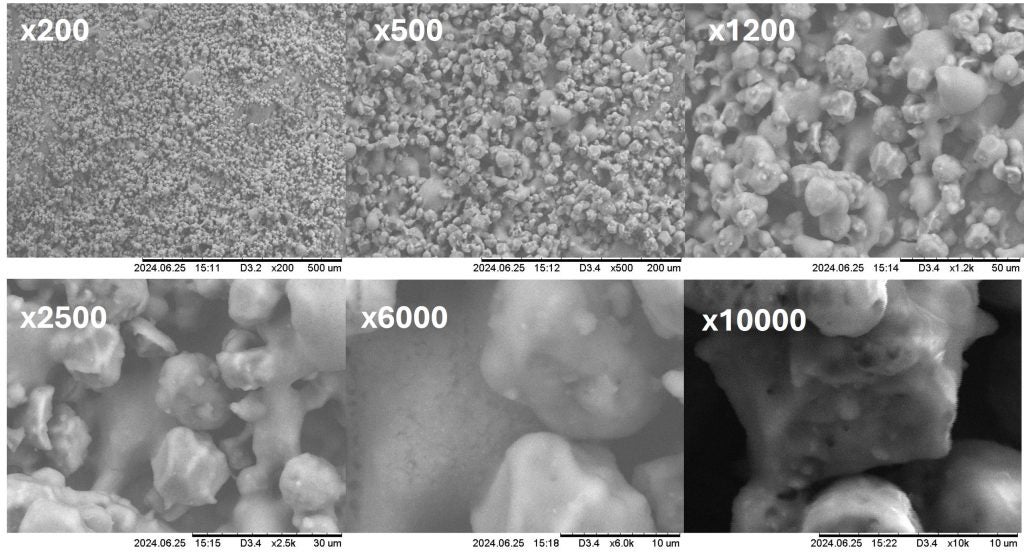
Scanning electron microscopy images were performed on all samples, while PSD measurements were done with the Malvern Mastersizer 3000. SEM images were taken a few days to a week later, and during this time the lyophilized L. plantarum samples were stored in 4°C fridge. This may have allowed the powder to agglomerate as seen on Figure 2. Due to the small size (0.9 to 1.2 µm wide and 3-8 µm long) single L. plantarum cells may not be clearly seen on those images but only solid agglomerates.4
Culture contamination control
Standard microscopy was performed to ensure no contamination occurred during the petri dish cultures. Those observations allowed us to ensure that the cultivated microorganisms were truly L. plantarum and to assess the overall health of the grinded sample by observing the morphology of the bacteria. A typical optical microscopy image may be seen on Figure 3.

Results and Discussion
Effect of Grinding Speed on PSD
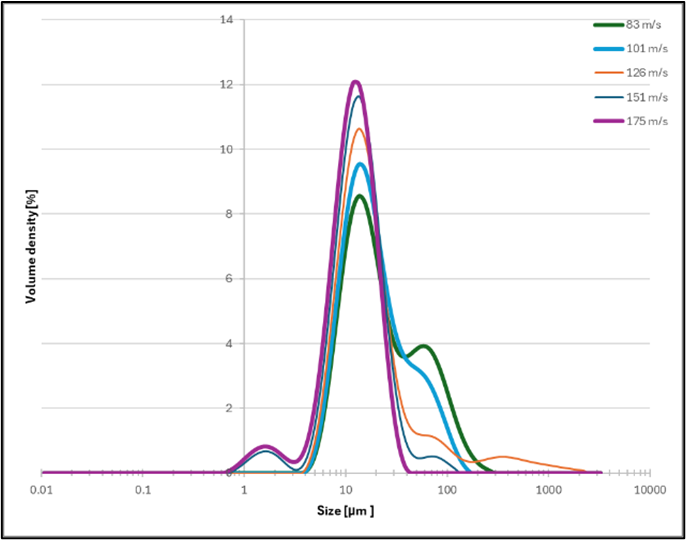
Grinding speeds significantly influenced D(50) and allowed the reduction of the amount of agglomerates, with higher speeds achieving finer particle distributions as seen in Figure 4. At 175 m/s, a D50 of 11.9 µm was recorded, representing a 67% size reduction compared to the control group. However, agglomerates were observed in samples processed at lower speeds (resulting in the formation of a secondary peak, Figure 2), and this was likely due to incomplete particle breakage.
Impact on Biological Activity
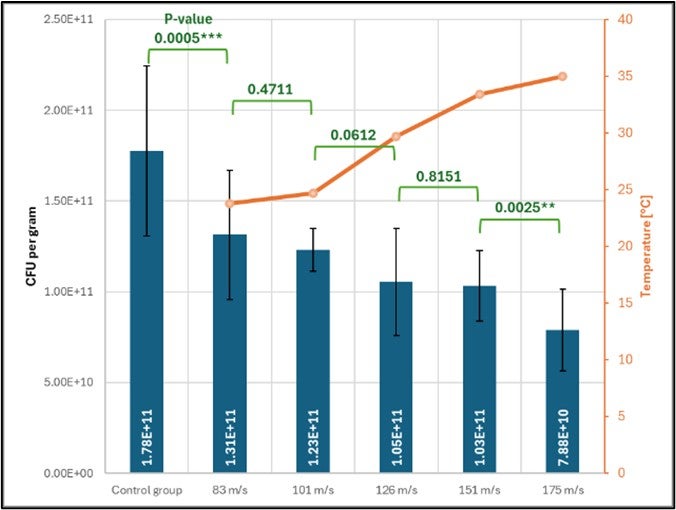
Grinding speeds exceeding 151 m/s resulted in significant reductions in CFU counts, with viability dropping by 56% at 175 m/s compared to unground samples. Statistical analysis showed no significant difference in CFU counts between 126 and 151 m/s, as demonstrated by a high P-value, indicating a possible optimization range where PSD can be improved without drastically compromising viability (see Figure 5). This optimal speed would allow further decrease of overall particle size, which would represent a notable gain of 14% in size reduction.
Mechanical stress Influence
Exit temperatures correlated with grinding speed, increasing from 23.8°C at 83 m/s to 35°C at 175 m/s. Elevated temperatures at the exit show that high mechanical stress is present during the grinding and likely contributed to decreased CFU counts at higher speeds. While localized heating in the grinding chamber remains a hypothesis, temperature monitoring at impact sites could further clarify these effects.
The PM-160 grinder’s modularity and controlled inert atmosphere offer promising scalability for probiotic production. The observed optimization range (126-151 m/s) suggests industrial feasibility for balancing particle size and viability, critical for effective probiotic formulations.
Conclusion
This work, conducted on the influence of milling on the biological activity of lyophilized microbial particles has provided insights into the effects of various grinding parameters on the viability of microorganisms.
The use of statistical tools, particularly Student’s T-test, allowed for a comparison point and a criterion for the discussion of empirical results. This statistical validation strengthens the reliability of the experimental results and emphasizes the necessity of careful parameter selection.
This project primarily focused on the impact of grinding speed on the colony-forming units (CFU) per gram of lyophilized Lactobacillus plantarum. The findings demonstrated that high-energy milling significantly affects the viability of microorganisms, with grinding speed playing a crucial role. The results indicate that grinding at higher speeds, such as 175 m/s for L. plantarum, leads to a significant reduction in CFU per gram. The mechanical stress induced by high-speed grinding causes considerable cell damage, thereby reducing the viability of the microorganisms. This finding underscores the importance of optimizing grinding parameters to maintain cell viability in industrial processes involving lyophilized microorganisms. Interestingly, results show that CFU per gram for L. plantarum didn’t show a significant change when grinded at 126 and 151 m/s, which indicates that speed could be increased to attain smaller particle size, resulting in an overall 14 % gain in size reduction.
References
(1) Hartmann, Yves. Influence of High-Energy Milling on the Biological Activity of Microbial Particles. BSc thesis, Haute école d’ingénierie et d’architecture de Fribourg, Fribourg, 2024.
(2) Student. The Probable Error of a Mean. Biometrika 1908, 6 (1), 1–25. https://doi.org/10.2307/2331554.
(3) Produits – FlexMill-Lab PM-160 – Frewitt – Votre partenaire pour le broyage et le traitement des poudres. Frewitt. https://frewitt.com/fr/produits/flexmill-lab-pm-160/ (accessed 2024-07-16).
(4) Üçok, G.; Sert, D. Growth Kinetics and Biomass Characteristics of Lactobacillus Plantarum L14 Isolated from Sourdough: Effect of Fermentation Time on Dough Machinability. LWT 2020, 129, 109516. https://doi.org/10.1016/j.lwt.2020.109516.


