Roche Diagnostics completed the construction and commissioning of a new manufacturing facility in Branchburg, New Jersey, in 2004. The manufacturing centre was constructed on the 63-acre Roche site in Branchburg; the existing 185,000ft² laboratories on this site have also undergone an extensive refurbishment as part of the project. The facility will manufacture and package Polymerase Chain Reaction (PCR) diagnostic kits for Roche Molecular Systems (RMS).
The new facility will consolidate the activities of three other sites operating in New Jersey (at Belleville, Totowa and Branchburg) to one centralised location. The project required an estimated investment from Roche of $155 million and employs up to 800 new personnel.
The new section of the facility has a total floor space of 285,000ft² and consists of a one-story state-of-the-art cGMP manufacturing centre building of 110,000ft², a short-term warehouse of 33,000ft², a kit assembly / packaging area of 22,000ft², a utility area of 22,000 ft² and a two-story office building of 33,000ft². The remaining space is taken by several Class 1000 cleanrooms, the central power plant, library / refectory, production testing laboratories and service areas.
PRODUCTS
The manufacturing centre will employ a patented technology for the development and production of biochemical reagents and the manufacture of diagnostic kits used by HIV patients to monitor the effects of daily medications.
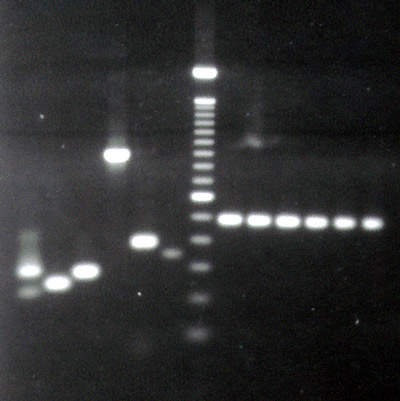
In the New Jersey manufacturing facilities, Roche Diagnostics produces 337 different diagnosis kits for the research, in vitro diagnostics (IVD) and blood screening markets. These are used for the detection and quantification of infectious diseases such as HIV, hepatitis and sexually transmitted diseases (STDs), as well as for blood screening, including the west nile virus kit 1, which is in clinical trials in the US and Canada. The production range also includes reagents for a variety of diagnostic platforms, including AmpliChip microarray technology. Overall, Branchburg produces around 130,000 kits per month, which are distributed worldwide. Kit volumes in Branchburg have grown about 20% during the last 18 months due to the introduction of new products and an increase and diversification in existing products.
CONTRACTORS
The complex was designed by the architect Einhorn Yaffee Prescott (EYP) of New York City, who was responsible for the overall master planning of the 63-acre campus. The car parking and road infrastructure of the facility was constructed and finished by Torcon Inc. The construction and engineering contractor was Fluor Daniel of Greenville, South Carolina. Johnson Controls were awarded a $4.6 million contract for the installation of building management and control systems and services.
Among the systems to be installed is a fully validated security system that complies with the US Food and Drug Administration (FDA) 21 CFR Part 11 regulation for electronic records and signatures. In addition to the security system, the contract includes the installation of closed-circuit television (CCTV), a state-of-the-art fire alarm system, and Metasys for Validated Environments (MVE), which is a Part 11 compliant building automation system (BAS).
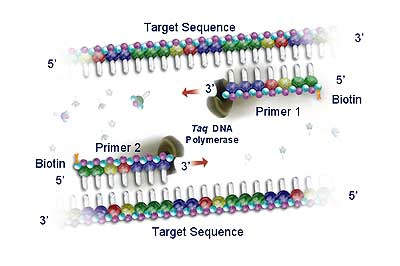
ROCHE PCR DIAGNOSTIC KITS
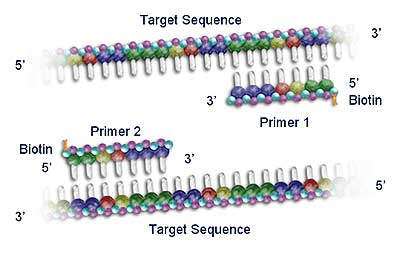
Roche acquired the rights to Polymerase Chain Reaction (PCR) technology in 1991, and since then has developed a commanding presence in the molecular diagnostics testing market. PCR is now the leading nucleic acid testing (NAT) technology in the world. The sensitivity of the technique allows clinicians to diagnose infectious agents, such as HIV and hepatitis viruses, at an earlier stage (by detection of minute quantities of their genetic material) and to monitor the progression of the disease and the response to therapeutic agents. Roche has over 130 US patents related to the PCR process.
Roche diagnostic kits use standardised PCR reagents with fully automated analyser instrumentation (including solid state detection chip technology such as the AmpliChip CYP450 microarray).