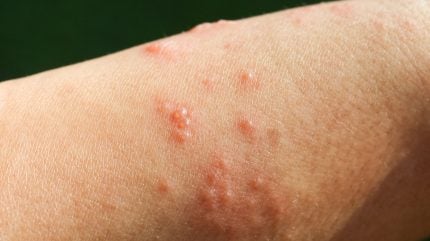
The US Food and Drug Administration (FDA) has approved Sanofi and Regeneron’s Dupixent (dupilumab) as a treatment option for adults with bullous pemphigoid (BP), a condition that predominantly affects the elderly.
BP is a chronic skin disease marked by severe itching, painful blisters, lesions and skin redness.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Dupixent’s approval was based on findings from the ADEPT Phase II/III trial, which assessed its efficacy and safety in comparison with placebo among adults with moderate-to-severe BP.
Participants were assigned either 300mg of Dupixent or a placebo in addition to standard-of-care oral corticosteroids (OCS).
Sanofi immunology and oncology development global therapeutic area head Alyssa Johnsen stated: “Until now, treating bullous pemphigoid was very challenging for elderly patients struggling with the debilitating impact of blisters and lesions, and potentially co-morbid conditions.
“By addressing two central drivers of the underlying type 2 inflammation that contributes to bullous pemphigoid, Dupixent is the first targeted medicine to allow patients the potential to achieve sustained remission and reduce itch. This approval in the US is important for the thousands of patients living with bullous pemphigoid, and we look forward to working with regulators around the world to bring this innovative medicine to more patients in need.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe priority review status granted by the FDA underscores the potential significance of Dupixent in enhancing both efficacy and safety outcomes for BP.
Dupixent is also undergoing regulatory evaluations internationally in the European Union, Japan and China.
It is being co-developed by Regeneron and Sanofi under a worldwide partnership agreement and has been studied across more than 60 trials in more than 10,000 subjects.
In February 2025, the FDA accepted Dupixent’s supplemental biologics licence application (sBLA) for priority review.




