
The healthcare landscape faces turbulent times. Regulatory pressures, challenges around demand forecasting and an influx of novel therapies are heaping pressure onto supply chains. A survey for GlobalData’s State of the Biopharmaceutical Industry Mid-Year Review quizzed hundreds of industry executives on their expectations for the year ahead; when asked to identify the trend they expected to have the greatest impact over the next 12 months, the largest proportion of respondents selected supply chain challenges.
According to GlobalData, global supply chain disruptions have become both worse and more frequent over the past decade. The timeline below outlines global supply chain disruptions since 1998 and likely causes of these disruptions. The disruption since the Covid-19 pandemic has been unprecedented. Geopolitical turbulence has reinforced the problem.
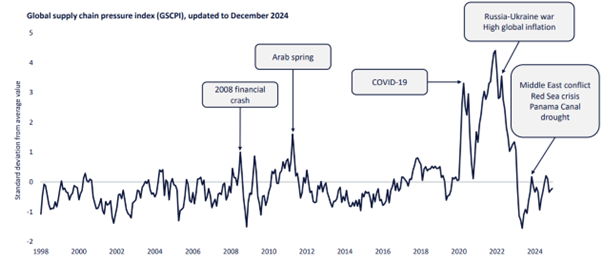
All these factors highlight the importance of utilizing data to predict potential supply chain issues before they arise. A data-driven approach can provide invaluable aid in planning and mitigating risks associated with supply chain disruptions.
However, reworking clinical supply chains can be complex and expensive, with no quick solutions. As firms navigate this evolving terrain, they face technological barriers that require an overhaul of their operational processes. To remain competitive and responsive amid rapid changes, biopharmaceutical businesses must embrace innovative solutions that enhance efficiency and agility.
Supply chain challenges can present opportunities for innovation. By leveraging AI and other novel technologies, firms can position themselves to effectively respond to two seismic challenges facing the biopharmaceutical world: navigating a deglobalizing trade environment and increasing demand for complex clinical trial materials.
Supply chain vulnerabilities
The fragility of global supply chains is a pressing concern. Factors such as geopolitical tensions, natural disasters and logistical bottlenecks can disrupt production and lead to shortages. Additionally, increasing emphasis on personalized medicine and specialty pharmaceuticals is shifting industry focus towards more tailored and complex treatment options, which further complicates supply chain logistics.
Biopharmaceutical firms will encounter a variety of specific challenges when attempting to transform their supply chains. These challenges can be broadly categorized into regulatory, logistical, technological and market-driven factors.
Regulatory complexity
One of the most significant challenges is navigating the regulatory landscape, which has become increasingly complex due to geopolitical tensions and divergent regulations between countries. For instance, the impact of Brexit has led to differing regulations between the UK and EU, complicating the transport of pharmaceutical materials across borders. In the US, announcements from the White House regarding “most-favoured-nation” drug pricing, alongside drug price constraints in the Inflation Reduction Act (IRA), are squeezing manufacturer profit margins.
This is just one of the pressures manufacturers face. Regulatory headaches around clinical supplies aren’t going anywhere. Importing, labeling, storage, and overall management of clinical supplies within the pharmaceutical industry are governed by extensive regulatory frameworks. These regulations are crucial for ensuring product safety, efficacy and compliance throughout the product lifecycle – but can also impede commercialization. Variance across jurisdictions is wide; noncompliance in just one can result in severe penalties, including the refusal to approve facilities for manufacturing or the products themselves.
Trade tensions
Companies are also facing increased risks associated with dependencies on single regions or vendors for input materials, which can threaten supply chain stability. These challenges are exacerbated by broader economic factors like inflation and trade tensions, which further complicate the procurement and distribution of clinical trial materials.
Time and temperature sensitivity
Biopharmaceutical products, particularly cell and gene therapies, often have strict requirements for storage conditions, necessitating robust cold-chain logistics. These therapies are not only time-sensitive but many also require ultra-cold storage solutions, posing significant challenges for maintaining the integrity of the supply chain.
Technology
While there is a growing appetite for digital transformation and real-time data tracking within supply chains, integrating these technologies can be challenging. Firms must invest in new systems and training to adapt to the evolving technological landscape, which is essential for enhancing supply chain efficiency and sustainability.
Workforce and skill shortages
The biopharmaceutical industry faces a shortage of qualified personnel, which can impact the ability to scale production and maintain quality control. This shortage is particularly acute in specialized areas such as cell therapy administration, where a highly skilled workforce is essential.
Streamlining clinical supply chain management
Addressing these challenges requires innovative solutions and strategic investments. Where should biopharmaceutical businesses start?
AI’s role will be pivotal. By automating supply chain tasks, intelligent automation systems can monitor inventory levels in real time, manage suppliers and even optimize product sourcing. The adoption of machine learning (ML) tools – which can analyze vast datasets to turbocharge the efficiency of ordering processes – is improving clinical and commercial supply chains across various metrics, including costs and time. As the volume of such complex datasets grows, AI can step in to drill further into these datasets, identifying patterns and trends that can inform strategic decisions.
This capability is already being used in drug discovery and development. AI can significantly reduce the time required to identify drug targets and streamline research processes. Similar applications are possible across supply chains; AI-driven predictive analytics can create optimization plans and schedules, helping organizations to anticipate issues before they arise. And AI tools can detect anomalies in data, enabling proactive adjustments in supply chain operations to both minimizing disruptions and maintain compliance with regulatory standards.
A common headache faced in clinical trials is drug supply forecasting. Getting it wrong can result in either overproduction or critical shortages at trial sites. It is a perfect use-case for AI; trailblazing tools can analyze real-time enrollment and patient dropout rates, and, combined with historical data from similar trials, generate precise demand forecasts to significantly lower costs and reduce wastage. Further, AI’s ability to simulate multiple scenarios based on different variables allows firms to make rapid adjustments when faced with freak events like natural disasters. It means the correct product and quantities can always reach the correct trial sites when needed, whatever bumps they encounter along the road.
Transforming clinical supply chains to support a smoother, more agile transition
When it comes to choosing an intelligent clinical supply management system, business decision makers need to look at how specialized tools can support a smooth, agile transition for their companies. Both SAP’s Intelligent Clinical Supply Management and Cell and Gene Therapy Orchestration solutions provide life science solutions that can optimize your supply chains today and futureproof them for tomorrow.
For instance, SAP Intelligent Clinical Supply Management simplifies clinical supply management by offering real-time insights, end-to-end visibility and improved collaboration. Integrating clinical trial parameters directly into supply chain planning and clinical trial management, enabling you to forecast clinical demand accurately and minimize waste. Clinical sponsors and CMOs can connect seamlessly over SAP’s Business Network platform, allowing them to exchange both drug and protocol information, while third-party logistics can be successfully integrated by using specific, GS1 industry standard, interfaces.
A more agile supply chain will respond to market fluctuations and regulatory requirements more quickly, and as the industry continues to evolve, companies that embrace these technologies will quickly take the lead in operational excellence and innovation.
For more information on how SAP can make your clinical supply management smoother and more agile, download the free paper below.


