In April 2019, Kite Pharma announced plans to develop a new chimeric antigen receptor (CAR) T-cell therapy production facility in Maryland, US.
The facility will manufacture various investigational T-cell receptor (TCR) therapies, including the commercially available personalised cancer treatment Yescarta® (axicabtagene ciloleucel).
The facility will generate employment for 400 people, when operational. An additional 500 jobs are expected to be generated in the future.
Kite Pharma is a subsidiary of biotechnology company Gilead Sciences.
Kite Pharma’s CAR T-cell therapy production facility location
The facility will be located on a 20.45-acre site at 9021 Bennett Creek Boulevard in the Urbana Corporate Centre in Fredrick County, Maryland. The site was purchased for $7.5m in November 2018.
Developed by Natelli Communities, the Urbana Corporate Centre is spread across 200 acres within the 1,100-acre Villages of Urbana community. The centre houses office spaces and a research and development (R&D) campus.
Located four miles from the border of the Montgomery County, the Urbana Corporate Centre leverages the Montgomery’s migrating population and attracts the workforce to Frederick.
Details of the cell therapy production facility
The site plan of the facility was approved by the Frederick County Planning Commission in September 2018.
The facility will consist of a two-storey office building with a gross floor area of 62,000ft². It will also comprise 217,000ft² of manufacturing, plant and shipping spaces, which are designed to expand the company’s manufacturing capabilities through innovation.
The state-of-the-art facility will enable the development of personalised CAR T-cell and TCR cancer immunotherapies, which require complex, controlled and multi-step processes.
Financing of Kite Pharma’s CAR T-cell therapy production facility
A conditional loan of $2m for the project was approved by the Maryland Department of Commerce through the Advantage Maryland Fund, which was previously known as the Maryland Economic Development Assistance Authority and Fund.
The Maryland Department of Commerce also granted $200,000 through the Partnership for Workforce Quality (PWQ) programme to support training.
Fredrick County is offering a commercial and industrial tax credit to Kite Pharma to encourage local manufacturing.
In addition, the company will receive job creation tax credits from the local and state governments such as Job Creation Tax Credit and More Jobs for Marylanders Tax Credit.
Technology used in the facility
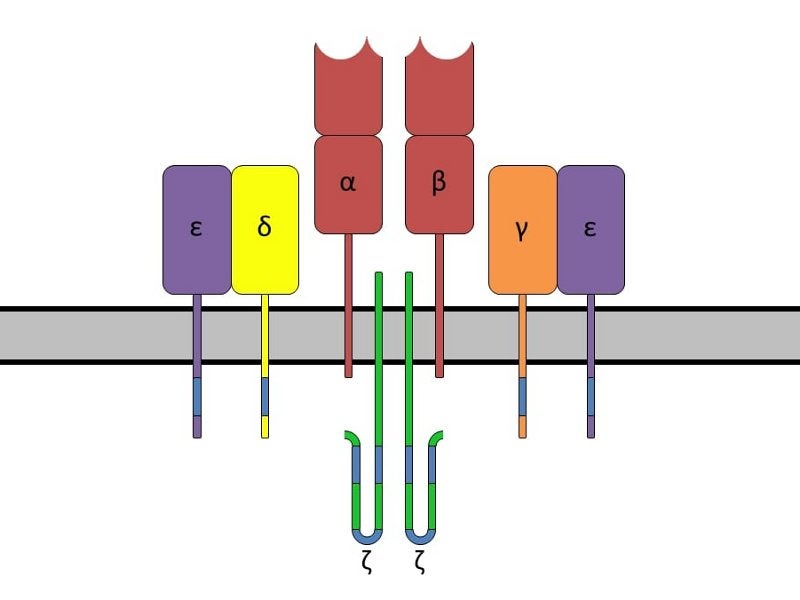
Kite Pharma develops T-cell based cancer immunotherapies by utilising CAR and TCR methods.
T-cells isolated from patients’ white blood cells are collected and engineered with either a CAR or TCR gene before being multiplied. The cells are then re-infused into the patient’s body to identify and kill cancer cells vigorously.
Yescarta is the company’s first commercially available CAR T-cell therapy for the treatment of large B-cell lymphoma. The company’s TCR programmes are based on the TCR-GENErator™ discovery platform, which was developed for rapid identification of TCR-based product candidates.
Marketing commentary on Kite Pharma
Headquartered in California, Kite Pharma designs and develops immune-based therapies for cancer treatment. The company was acquired by Gilead Sciences, a biotechnology company based in the US, for approximately $11.9bn in October 2017.
The acquisition added Kite’s CAR T-cell and TCR therapy platforms, as well as more than 700 employees to Gilead.
In June 2016, the company opened a 43,500ft² state-of-the-art T-cell therapy manufacturing facility in El Segundo, California. The firm is currently developing a 117,000ft² facility in Hoofddorp, Netherlands.
Kite Pharma also leased a 26,000ft² facility in Gaithersburg, Maryland, to support the development of adoptive cell therapies that target patient-specific tumour neoantigens found on the cancer cells surface. The work will be carried out along with the National Cancer Institute under a cooperative R&D agreement (CRADA).