After four years of inactivity, the US Food and Drug Administration (FDA) approved three novel products for hereditary angiooedema (HAE) in 2025. This bumper year increased the total number of marketed innovator drugs within the US to 11, introducing new mechanisms and modalities which broaden the treatment strategies available to patients.
HAE is a potentially life-threatening inherited disease. This disorder is characterised by recurrent and severe episodes of swelling in various parts of the body, including the hands, feet, genitals, stomach, and face or throat, which can prove fatal. This is a rare condition, which is estimated to affect only 7,000 people in the US, approximately.
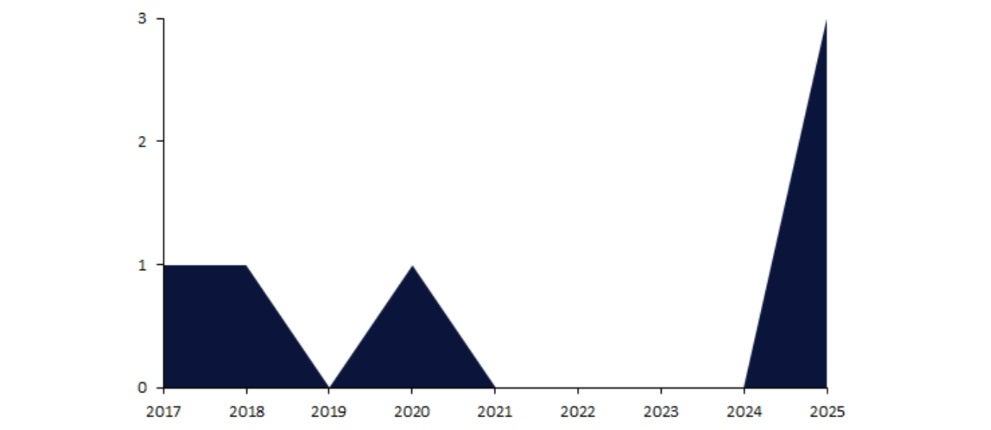
FDA approvals in HAE were sparse before 2025, with only one each in 2017, 2018, and 2020. This was followed by a lack of approvals for four years (2021-24); a drought where patients were waiting for novel mechanisms to break through.
Yet, in 2025 so far, the FDA has approved three drugs for HAE (Figure 1). This bumper period of activity has effectively reshaped the treatment landscape for HAE patients in under nine months.
Three FDA approvals for HAE in 2025 not only increase the total number of marketed products in the US by 38%, but also introduce new and effective modalities, which revolutionise HAE management.
CSL Behring received the first FDA drug approval in HAE with garadacimab on 16 June 2025. This milestone marked the backing of a new biological mechanism, as this is the first approval of a drug targeting coagulation factor XII. Since this protein plays a major role in initiating the swelling cascade, its inhibition offers a direct way to block the onset of attacks. As such, this approval marks a strategic move towards earlier intervention in this disease pathway.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataJust weeks later, KalVista secured FDA approval for sebetralstat (Ekterly), the second oral drug for HAE, on July 3. Unlike BioCryst’s oral drug berotralstat hydrochloride, which is taken daily for prophylaxis, Ekterly is administered following the onset of symptoms, offering patients an ‘on-demand’ treatment for the first time. This approval represents a meaningful advancement in convenience and flexibility for patients requiring rapid and effective relief during acute episodes.
The third and most recent approval came on 21 August, when Ionis Pharma’s donidalorsen sodium became the first antisense oligonucleotide approved for HAE. This prophylactic treatment offers patients an extended dosing interval of every four to eight weeks, much longer than the every-two-week schedule of other preventive therapies. This approval not only introduces a novel modality, but also requires less dosing, potentially improving patients’ quality of life.
After four years of regulatory silence, the surge of FDA activity in 2025 has redefined the therapeutic landscape for HAE, with three novel approvals in a year increasing the total marketed drug count by 38%, to 11. The new drugs introduce a variety of distinct treatment modalities which offer new and unique ways of managing the disorder. The diversification of products provides patients with greater flexibility in terms of prophylactic and/or acute treatments, which reduces the burden of the disorder, enabling patients to match treatment strategies to their individual lifestyles.