The use of real-world evidence (RWE) began gaining traction decades ago, primarily as a response to the limitation around traditional clinical trials’ ability to provide comprehensive and timely insights into treatment effectiveness, especially beyond controlled environments. Given its ability to offer these insights, RWE has become a more crucial tool in decision making, improving both efficiency and a patient-centered approach in healthcare.
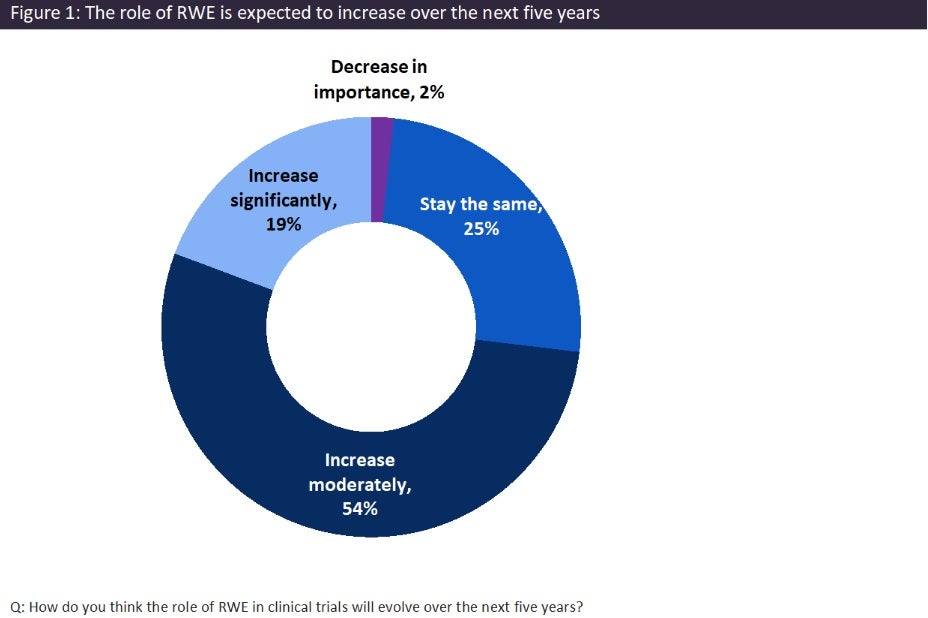
To gather physicians’ opinions and perspectives on the future of RWE, leading data and analytics company GlobalData surveyed 290 healthcare professionals (HCPs) from April to July 2025. When asked about changes in the role of RWE in clinical trials, the majority of the physicians believed that it would moderately (54%) or significantly (19%) increase (Figure 1). This indicates the perceived growth of RWE’s value in clinical trials. This shift also highlights the broader trend in healthcare towards more personalised and evidence-based decision making, which will help to accelerate drug development, improve treatment outcomes, and refine the future of the healthcare.
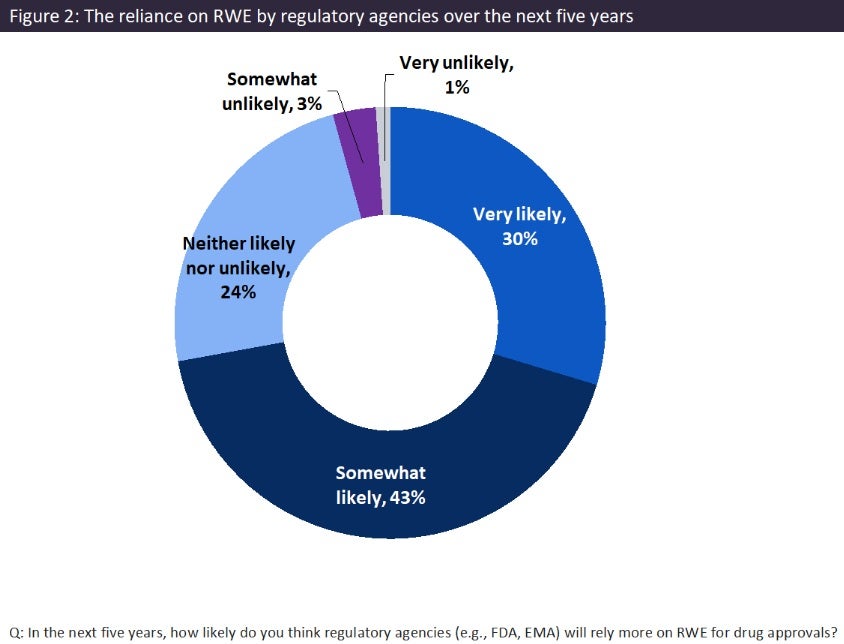
Surveyed physicians also believed that the role of RWE would grow in regulatory drug approvals, with 73% of HCPs believing that the role will be somewhat or very likely to increase (Figure 2). Recent regulatory developments also indicate this opinion. In the US, the Food and Drug Administration’s (FDA) Prescription Drug User Fee Act VII, which runs through 2027, outlines the use of RWE in drug development and post-marketing surveillance, allowing for more flexible approval pathways. This emphasises the FDA’s commitment to realise the full potential of fit-for-purpose real-world data to generate RWE. However, in Europe, the European Medicines Agency’s (EMA) framework, which was updated in 2023, continues to evolve, paving the way for more robust RWE integration into decision making. Ongoing frameworks such as the Data Analysis and Real World Interrogation Network – which delivers RWE from across Europe on diseases, populations, and the uses and performance of medicines – also help to enhance the role of RWE by further integrating it into drug development and regulatory decisions.
The integration of RWE in clinical trials and regulatory approvals is set to grow substantially. As regulators and healthcare communities, including physicians, continue to embrace and trust in RWE, it will likely become a standard component in supporting faster and more efficient processes.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData




