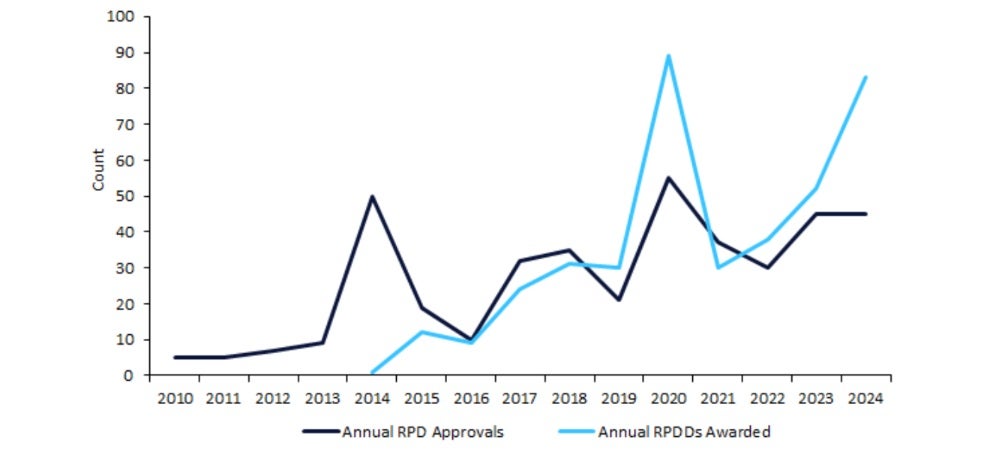
Due to concerns surrounding its effectiveness, the US Food and Drug Administration (FDA) ended the Rare Pediatric Disease Designation (RPDD) programme in late 2024. Following this programme’s introduction in 2014, the FDA oversaw a surge in drug approvals for rare pediatric disorders (RPDs). From 2016 to 2024, a positive trend in RPDDs awarded was mirrored by a general increase in RPD approvals. These findings suggest that the implementation of RPDDs positively impacted the RPD market. However, their ultimate impact has received scrutiny.
Given the small patient populations and limited financial incentives, RPDs have been historically overlooked in drug development. In July 2012, the FDA launched the RPDD. This designation was awarded to drugs that were being developed for rare and serious diseases that primarily affect individuals younger than 18. The RPDD provided financial incentives to pharma companies developing these treatments. If a drug with RPDD was subsequently approved, the company received a Priority Review voucher. This voucher offered a streamlined and prioritised FDA review for a future drug or could be sold for as much as $350m.
Due to concerns over its effectiveness, the US Congress did not renew the RPDD programme and began to close the programme in late 2024. Figure 1 (above) highlights trends in RPD approvals from 2010 to 2024, alongside annual RPDDs awarded by the FDA, which commenced in 2014.
Between 2010 and 2013, annual FDA approvals for RPDs remained relatively low and stable, at an average of 6.5 per year.
In 2014, the FDA began awarding RPDDs. This same year, the FDA awarded 50 approvals for RPDs, at a five-fold increase from 2013. However, only one of the 50 RPD approvals in 2014 was linked to an RPDD. Thus, the 2014 surge in RPD approvals was due to a broader FDA initiative to prioritise paediatric diseases, as opposed to the RPDD programme having a direct impact.
In 2016, only ten RPDs were approved by the FDA. Despite various peaks and troughs between 2016 to 2024, this period saw an overall positive trend for annual RPD approvals, growing at a compound annual growth rate (CAGR) of 16.2%. A peak of RPD approvals came in 2020, with the FDA handing out 55 approvals. Over this period, trends in RPDD awarding were similarly positive, at a CAGR of 24.9%. Like RPD approvals, 2020 was a peak year for RPDDs with 89 designations awarded, an almost three-fold increase from the 30 designations awarded in 2019. This paralleled growth suggests that the RPDD programme may have played a reinforcing role in the elevated industry focus on rare paediatric therapies.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThese findings suggest that the FDA began placing significantly greater emphasis on RPD drug development in 2014 through the introduction of the RPDD programme and a surge in RPD approvals. However, the direct impact of RPDDs on the market remains unclear. Nevertheless, increases in both RPDDs awarded and RPD approvals from 2016 suggest that the FDA’s focus continued, with the offering of financial incentives potentially stimulating enhanced efforts from drug developers. As the RPDD programme closes down, the future RPD landscape remains uncertain. New policy efforts must be introduced to ensure the trend of increasing RPD treatments and approvals continues for years to come.