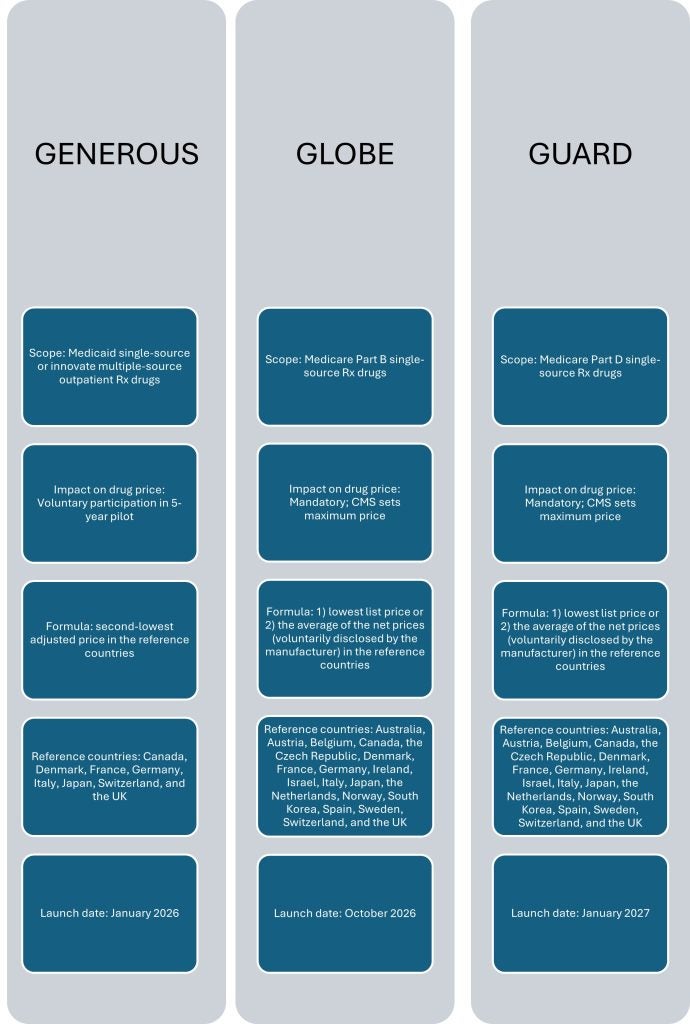
Just before Christmas 2025, the US Centers for Medicare and Medicaid Services (CMS) unveiled two pricing models based on the use of international reference pricing (IRP). These models—nicknamed GLOBE and GUARD—advance President Donald Trump’s most favored nation (MFN) price-setting agenda for medicines, and represent a much more serious threat to pharmaceutical industry profits in the US and to patient access to medicines globally than the GENErating cost Reductions fOr U.S. Medicaid (GENEROUS) model announced in November.
The GENEROUS model was already a threat. It envisaged taking the use of IRP in the US far beyond the type of IRP Trump attempted to implement during his first term in office. GlobalData’s detailed strategic reports on the MFN executive order and on the GENEROUS model examined how some of the known adverse effects of IRP will be unleashed in terms of pricing pressure, lost revenues, and delayed or cancelled drug launches, while outlining the strategies pharma companies could employ to reduce such impacts. However, the GENEREOUS model had one key advantage over the new models: it was voluntary for pharmaceutical companies. It also had a narrower application by targeting Medicaid drugs only.
The two newly released IRP models are instead mandatory and target the prices of drugs sold under the larger Medicare programs for over-65s. GLOBE, whose full name is Global Benchmark for Efficient Drug Pricing, targets Medicare Part B drugs (essentially prescription [Rx] drugs administered in doctors’ practices or clinics) and comes into effect in October 2026. GUARD (short for Guarding US Medicare Against Rising Drug Costs) will apply to Medicare Part D—i.e., all outpatient prescription drugs covered under the reimbursement program for seniors—and is set to launch in January 2027. Crucially, these two models will be mandatory for all companies that have not entered a voluntary MFN agreement with the US government. Medicines whose prices are already negotiated and cut under the Inflation Reduction Act (IRA) provisions will also be spared. However, these are small exemptions. For all intents and purposes, IRP is set to become the main pricing mechanism for all originator/single-source prescriptions drugs covered under the largest publicly funded reimbursement program for prescription drugs in the US. And with this, there will also come a heightened risk of price leakage from Medicare to private sector reimbursement plans for medicines—further intensifying the pressure on pharma companies’ bottom lines.
Figure 1: Selected elements of US IRP system under the three models
The pre-Christmas announcement was the exact opposite of a Christmas present for a weary pharma industry that has done its best to appease an increasingly hostile and belligerent Trump administration. There have been numerous industry attempts—examined in GlobalData’s Price Intelligence (POLI) and World Markets Healthcare (WMH) daily analyses—to offer voluntary concessions in an effort to avert the introduction of a formal, mandatory IRP system in the US. The unveiling of GLOBE and GUARD suggests that such attempts have failed and the US is set to move towards the formal use of IRP.
The pharma industry does, however, still have tools at its disposal to persuade the Trump administration to modify its IRP plans. Pharmaceutical companies are already doing certain things that can mitigate the revenue hit—most notably by employing direct-to-consumer (DTC) sales techniques. DTC selling allows companies to bypass the wholesale and retail sectors, allowing the marketing authorisation holder to retain a greater share of revenues from each product.
However, there is more that can be done, specifically in relation to IRP and utilising its elements to reduce revenue headwinds, both in the US and in other countries, whose price levels will be impacted by the US’ use of IRP. MFN plans may be new for the US, but IRP has been used widely around the world. More than 75 countries already use IRP to either directly set drug prices or as one of several inputs into price negotiation. GlobalData’s detailed IRP country profiles, examining every aspect of the particular country’s IRP system elements, provide a useful indicator of how the US IRP system may evolve. POLI data on launch sequencing meanwhile allows precise measurement of IRP’s impact on company strategies. IRP reform impact can be separated from that of concurrent pricing and reimbursement reforms and from the launch delays triggered by the pricing and reimbursement approval process in each market.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAnother crucial element that can help with pharma industry advocacy efforts is the experience with drug shortages and examples of how different countries around the world have amended their pricing and reimbursement policies, including the use of IRP, in response to shortages. There is ample evidence of such impacts in GlobalData’s 25 years’ worth of daily analyses, which can support advocacy efforts, while data on shortages at the drug pack level is captured in POLI. Just two pertinent examples to mention here are the decision in the Netherlands to cap price cuts triggered by its IRP reference countries basket reform in 2020, and the delay to Canada’s IRP reform following industry pushback and concerns about drug shortages. Canada not only delayed changing its reference country basket by several years, but, in an unexpected twist, announced in 2025 that it will reference the highest price among its reference countries instead of the median.
Policy changes are possible when the adverse effects of IRP, including pressure on drug supply, become evident to policy-makers.
This article is produced as part of GlobalData’s Price Intelligence (POLI) service, the world’s leading resource for global pharmaceutical pricing, HTA and market access intelligence integrated with the broader epidemiology, disease, clinical trials and manufacturing expertise of GlobalData’s Pharmaceutical Intelligence Center. Our unparalleled team of in-house experts monitors P&R policy developments, outcomes and data analytics around the world every day to give our clients the edge by providing critical early warning signals and insights. For a demo or further information, please contact us here.