Nutraceuticals – foods that contain health-giving additives – have shown promising trial results for a range of diseases, but a lack of co-operation between the regulatory agencies of different regions is restricting their availability, which in turn is discouraging large multinationals from investing in them.
Well known nutraceutical products include Yakult and Omega-3 supplements, although many varieties exist.
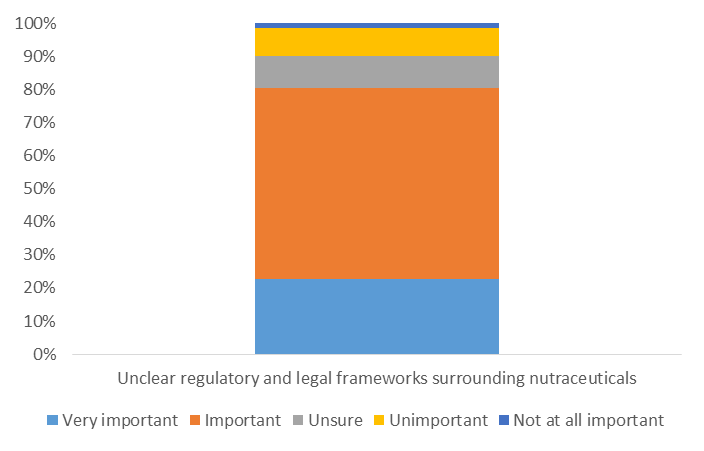
In a recent survey, GBI Research found that 81% of professionals who worked in the food and pharmaceutical industry but were not involved in nutraceuticals considered unclear regulatory and legal frameworks a key restraint to the growth of the market.
And even among those who worked in the nutraceutical industry, 72% shared this opinion.

The regulatory frameworks of Europe and the US, although similar, have different product classes and definitions, and lack precise definitions of the classes of products that nutraceuticals can occupy.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThis leads to situations like those seen with Deplin and Souvenaid, where Deplin is unavailable in Europe, and Souvenaid is unavailable in the US – despite these products demonstrating clinical efficacy in depression and Alzheimer’s disease, respectively.
Regulatory clarity and synergy would make more products available to patients, increase revenue for manufacturers, and incentivise the development of more high-quality nutraceuticals, which would enable healthcare systems to provide more treatment options at lower costs.