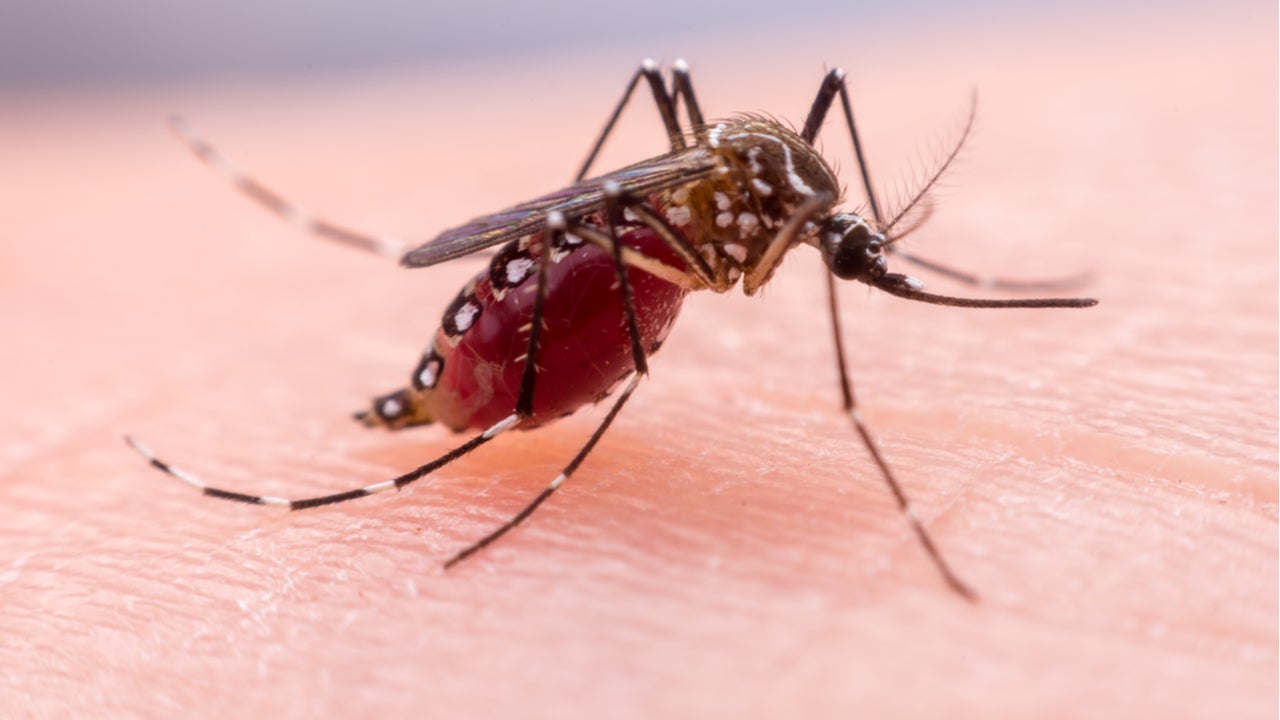
Zika virus overview
Zika virus is a member of the Flaviviridae family of viruses, which also include Yellow fever, West Nile, and Dengue viruses.
The virus is primarily spread by Aedes mosquitoes such as A. albopictus and A. aegypti. It can also transmit through sexual contact and from a pregnant mother to the foetus.
The virus is largely prevalent in the equatorial and other mosquito-rich regions.
Symptoms and disease severity
The Zika virus infection is asymptomatic or causes mild symptoms. It is generally compared with mild dengue and causes fever, head and joint aches and body rash. The disease, however, may lead to two severe conditions namely Guillain-Barré syndrome and birth defects.
Guillain-Barré syndrome is a muscle weakness caused when the immune system damages nervous tissues, sometimes causing paralysis. The incidence rate of this condition is very rare raising doubts on whether Zika infection is associated with Guillain-Barré syndrome.
The Zika infection is associated with neurological birth defects as the virus can spread from a pregnant woman to her baby. The most common birth defect is microcephaly.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
By GlobalDataZika virus epidemic: 2015–2016
Brazilian physicians identified a potential Zika virus outbreak in the country in 2014 , which was deemed an epidemic from 2015-2016. The outbreak was contained from late 2016 following aggressive mosquito control measures.
Most of the cases were reported from South America, with Brazil accounting for majority of the cases.
Thousands of birth defects linked with Zika were later identified triggering awareness about the outbreak. Brazil reported more than 137,000 confirmed Zika infection cases, 11 fatalities and 2,952 confirmed congenital syndrome associated with Zika.
Puerto Rico, Mexico and Colombia also reported a number of cases.
Response to the outbreak: Control and containment measures
The authorities adopted aggressive mosquito control policies to control the outbreak, as it is primarily spread by Aedes mosquitoes.
The measures taken included the use of ovitraps, which are devices where mosquitoes can lay their eggs, but prevents the developing larvae to escape. The authorities also used self-limiting mosquitoes, which are sterile male mosquitoes that cannot transmit pathogens. They are approved for use in certain countries and were effective in reducing mosquito populations.
Further, the authorities banned standing water reservoirs, a key breeding ground for mosquitos.
Separately, several high-level public measures were taken including the issuance of travel warnings to countries affected by Zika virus and encouraging people to wear protective clothing and mosquito repellent. Several countries also recommended their population to defer pregnancy due to the increased risk of microcephaly.
Zika virus vs COVID-19: Disease and outbreak comparison
A comparative study indicates that COVID-19 is more contagious, widespread and deadly compared to Zika infection.
Zika
The first illness was reported in March 2015 during the 2015-2016 epidemic and the first case was confirmed a month after in April. Brazil’s Ministry of Health announced the circulation of Zika virus in May 2015 and subsequently, in July, associated neurological disorders in new-borns were confirmed.
The World Health Organization (WHO) declared the outbreak a public health emergency in February 2016. The emergency was lifted in November of the same year.
The symptoms of Zika virus are generally mild. Patients usually complain of fever, rash and body aches. The rate of transmission is low. The mortality rate is also very low and stands at around 0.01%.
COVID-19
The first case of atypical pneumonia was reported by Chinese state media on 31 December 2019 and was then reported to the WHO. The virus was named SARS-CoV-2 on 11 February 2020 and the authorities confirmed person-to-person transmission.
The WHO declared COVID-19 as a global pandemic on 11 March 2020. The number of COVID-19 cases crossed one million by 3 April 2020, with more than 58,000 fatalities.
Several countries across the world entered lockdown to curb the spread of the disease.
The most common symptoms in COVID-19 are fever and dry cough. Around 80% of the cases are mild, while complicated ones require special treatment including ICU admission.
The mortality rate for COVID-19 is around 1%-3%, even though the actual rate is yet to be determined due to underdiagnosis of positive cases.
Pharmaceutical industry response
Vaccines
Zika has no approved therapeutic, which triggered robust enthusiasm initially. More than 75 vaccine candidates entered the developmental phase at the start of the outbreak. The developers included small biotechs as well as established names. A majority of the agents, however, did not enter the clinical phase due to waning interest and successful containment of the outbreak.
Therapies
Around 70 therapies were being developed for Zika virus initially with treatments ranging from small molecules to therapeutic antibodies and antisense oligonucleotides. However, no agent was successfully developed despite the strong pipeline.
Current state of Zika treatments and vaccines
The current management strategy focuses on symptomatic treatment and prevention of the disease as there are no approved therapeutics for Zika virus. Furthermore, a breakthrough in therapeutics in near future is very unlikely as very few agents are on the development horizon.
The most advanced agent is a single vaccine candidate from the NIAID, which is currently in Phase II trials. The developer interest is not expected to revive soon as Zika is not considered a public threat at the moment. Companies such as Sanofi have suspended promising programmes due to the withdrawal of funding and limited financial opportunity.
Future of Zika virus management and considerations for pharmaceutical development
The Zika virus outbreak was controlled rapidly, unlike the COVID-19 pandemic. The successful containment shrank the financial opportunities dissuading the pharmaceutical companies with the development of the therapeutic agents.
The rapid control also left very few patients to enrol for clinical trials of vaccines further increasing difficulties for the developers. It is unclear whether a vaccine will be included in routine recommendations due to high associated costs, even if a vaccine is approved. Any subsequent development, therefore, must be funded and driven by governmental or academic institutions.
Lessons learned and implications for COVID-19
Rapid response ability
The Zika outbreak demonstrated the ability and willingness to prioritise a developing health crisis and accordingly develop a response plan.
More than 100 vaccine and therapeutic agents entered development for Zika virus, underlining developers’ enthusiasm to capitalise on the opportunity presented by the outbreak. However, it also revealed the challenges and showed that it is important not to lose momentum before effective agents are found.
The pharmaceutical industry undertook a similar approach during COVID-19. The associated financial opportunities encouraged the companies to initiate the development of therapeutic agents. More than one hundred agents entered the developmental phase within two months of the virus’ discovery.
Drug repurposing
Antiviral developers adopted the drug repurposing strategy during the Zika epidemic that involves assessing the effectiveness of drugs already marketed or in the pipeline for treating another disease. It shortens the development timeline as the agent’s safety and tolerability levels are usually known.
The drug repurposing strategy met with a fairly high level of success during the Zika outbreak. Emricasan, which was being developed for treating a liver injury or fibrosis, and niclosamide, an approved treatment for tapeworms, were repurposed for Zika.
A number of pipeline candidates for other indications were tested for the novel coronavirus and the same technologies used to develop vaccines for Zika are being used for COVID-19.
Moderna’s mRNA-1273, for example, reached clinical trials in less than four months from the discovery of COVID-19.
Focus on profitability
The development of vaccine candidates for Zika came to standstill as financial opportunities dried up after the outbreak was contained. The absence of external funding further erased developer interest.
COVID-19, however, provides developers with increased financial opportunities and may require interventions for several years as it is unlikely to face a collapse as seen in the case of the Zika outbreak. The interest in vaccine development, however, is expected to subside after effective therapies and vaccines are developed.




Related Company Profiles
Aedes S.P.A
Moderna
Ministry of Health, Spain