Pharmatest offers specialised clinically predictive preclinical research services for the pharmaceutical industry.
Pharmatest provides full-service research solutions for oncology and skeletal diseases. The research models are designed to emphasise clinical relevance and predictivity.
Pharmatest can establish the proof-of-efficacy of drug candidates using cell culture assays and animal models.
The company has designed optimal study set-ups to test new drug candidates according to regulations of the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). It also offers specially designed studies for testing biosimilars and functional foods that require less extensive requirements.
Cancer models
The company specialises in full-service efficacy testing of new cancer drug candidates. In vitro studies demonstrate what cancer cell lines are affected by test compounds and efficacy is confirmed in vivo in the company’s animal models using the same cell lines.
In vitro assays for cancer screening applications
Pharmatest’s in vitro cancer cell assays, including cell viability and cell invasion, are rapid and reliable screening tools for cancer drug discovery. They are available in automated high-throughput screening (HTS) platforms.
Cancer cell invasion is measured using the BD BioCoat FluoroBlok invasion system. Cell migration capacity through the Matrigel layer is determined, while cell invasion is quantified by post-labelling cancer cells with a fluorescent dye.
Clinically predictive cancer models
Pharmatest specialises in clinically predictive orthotopic and metastasis models of breast, prostate and pancreatic cancer, as well as multiple myeloma. They provide the proper tissue microenvironment, which is crucial since it is known to affect tumour growth, metastasis and drug resistance.
The clinical relevance and predictivity is relevantly increased over traditionally used subcutaneous models that lack the proper microenvironment.
Orthotopic and metastasis models
Orthotopic breast cancer models are available with human estrogen receptor positive MCF-7 cell line, triple negative human MDA-MB-231(SA) or mouse 4T1 cell lines. Tumour growth is monitored by measuring dimensions of the tumour at intervals.
Pharmatest’s intracardiac breast cancer bone metastasis models are predictive models of cancer-induced bone disease with radiography and dynamic fluorescence imaging to reveal illness progression.
The company provides orthotopic prostate cancer models with the androgen receptor positive, and PSA producing LNCaP cell line.
Models are also available with the androgen receptor negative PC-3 cell line that develops metastases to prostate-draining lymph nodes, in addition to intracardiac and intratibial models of prostate cancer bone metastasis, where radiography reveals the progression of bone metastases.
Pharmatest offers a mouse model of multiple myeloma that mirrors clinical conditions of myeloma bone disease in patients. The model responds to treatment and is ideal for testing efficacy of drug compounds against multiple myeloma, specifically active myeloma, which is characterised by osteolytic lesions. It involves inoculation of mouse multiple myeloma cells into the tail vein of immunocompetent mice.
Pharmatest also supplies an orthotopic pancreatic cancer model, which is suitable for testing efficacy of drug compounds against primary tumour growth and responds to treatment. The model involves a surgical procedure where human pancreatic adenocarcinoma cells are inoculated into the pancreas of immunodeficient mice.
Skeletal disease model
Pharmatest’s clinically predictive skeletal disease models include bone cell culture assays and animal models of osteoporosis and osteoarthritis, which can also be used for bone safety testing.
In vitro bone cell cultures
Pharmatest’s in vitro bone cell assays have been designed for pharmacodynamic testing of new therapeutic agents against osteoporosis and cancer-induced bone disease before entering animal studies. Separate assays are available for testing effects on differentiation and activity of bone forming osteoblasts and bone resorbing osteoclasts.
In vitro osteoblast assays involve culturing mesenchymal progenitor cells that are differentiated into mature osteoblasts. In vitro osteoclast assays involve culturing bone marrow derived osteoclast precursor cells that are differentiated into mature bone-resorbing osteoclasts.
Osteoporosis and osteoarthritis models
Pharmatest’s in vivo models of osteoporosis include:
- Rat and mouse ovariectomy (OVX) models of postmenopausal osteoporosis
- Rat orchidectomy (ORX) model of male osteoporosis
- Mouse model of glucocorticoid-induced osteoporosis (GIO)
According to FDA and EMA regulations, all new therapies for postmenopausal osteoporosis should be tested in the rat OVX model.
The company’s in vivo models of osteoarthritis include:
- Monoiodoacetate (MIA) model, where OA is caused chemically by intra-articular injection of MIA
- Medial meniscal tear (MMT) and medial collateral ligament transection (MCLT), where OA is caused surgically by MMT and MCLT
- Anterior cruciate ligament transection (ACLT), where OA is caused surgically by ACLT
- ACLT and partial medial meniscectomy (pMMx), where OA is caused surgically by ACLT and pMMx
- pMMX and ACLT models in rabbits
Bone analysis services
The following bone analysis methods are included in different disease models and are also offered as a standalone service:
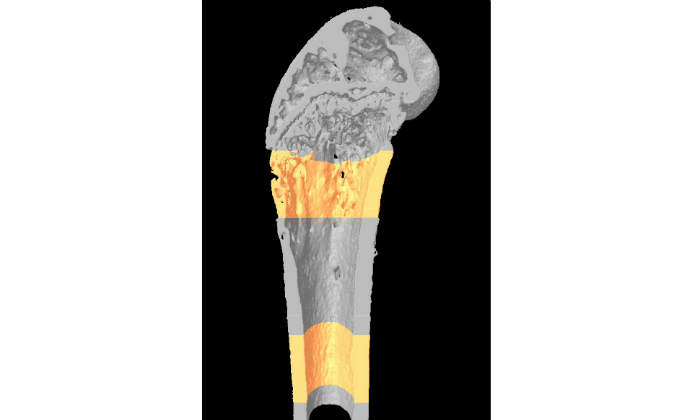
- Micro-computed tomography
- Peripheral quantitative computed tomography
- Radiographic imaging and image analysis
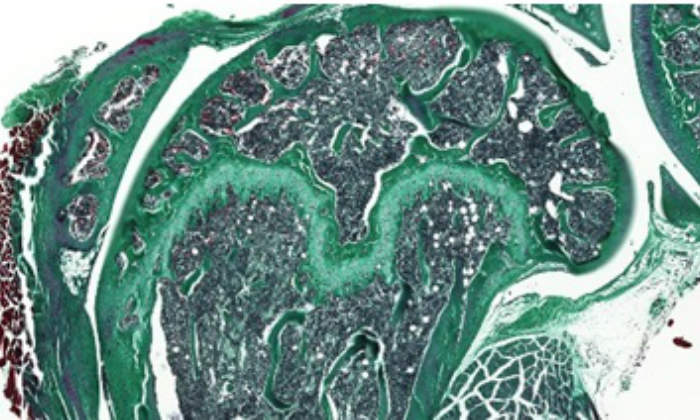
- Full-service bone histomorphometry
- Processing tissue samples for histological analysis
- Bone ash weight and biomechanical testing
- Biochemical markers of bone turnover in serum
- Gene expression analysis using microarrays and bioinformatics services