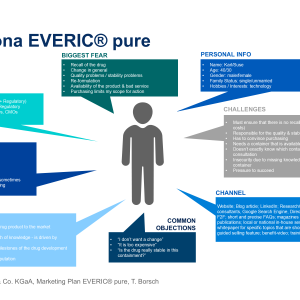
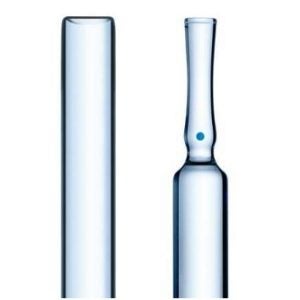
SCHOTT Pharmaceutical Packaging Releases New White Paper
SCHOTT Pharmaceutical Packaging has released a new, free-to-download white paper which explores ways to minimize the delamination risk of glass containers.
There has been a recent spike in market recalls for injectable drug products due to visible flaky particles associated with ‘glass delamination’. It is important for both drug product and container manufacturers to understand the underlying mechanisms, to test for the potential risk, and to control drug container interactions to provide patients with safe medicine.
The article, first published in the January 2012 edition of the PDA Letter, is now available to download here.