Cannabinoids, the class of chemical compounds found in the cannabis plant, represent a potential drug development treasure trove. Different cannabinoids have been shown to have pain-relieving, anti-spasmodic, anti-cancer and anti-inflammatory properties. These compounds can be used to stimulate or suppress appetite, reduce nausea or even as an antioxidant. Their usefulness for certain sufferers of multiple sclerosis (MS), Aids and cancer patients undergoing chemotherapy, among others, is beyond doubt.
Despite the inherent pharmacological value of these materials, this area has not been as intensely investigated as one might suspect. This could be because of a lingering stigma surrounding cannabinoids and their perceived association with a recreational drug that is illegal in most countries.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
THC, the most well-known cannabinoid (and the only one with a psychoactive or “high-inducing” effect), is only one of a large number of pharmacologically active cannabinoids, but its coverage has far outstripped that of any other. Synthetic medicines that mimic the effects of cannabinoids, such as Marinol and Cesamet, are available in some countries, but there is only one licensed prescription medicine derived directly from the cannabis plant.
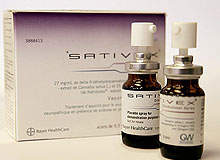
Sativex: the world’s first cannabis-derived medicine
Sativex is an oral spray developed by UK-based pharmaceutical company GW Pharmaceuticals, derived from the cannabinoids THC and CBD (cannabidiol). Sativex is now fully licensed in the UK, Spain, Canada and New Zealand for the treatment of muscle spasticity in MS patients. It is also approved in Canada for the reduction of cancer pain and neuropathic pain, and is in various stages of the approval process in the European Union and the US.
For Dr Geoffrey Guy, executive chairman at GW Pharmaceuticals, the main aim was to create a drug with the widest possible “therapeutic window” – the dosage range at which a drug is effective without causing unacceptable side effects. This was achieved both in the delivery method and in the combination of cannabinoids used. Sativex’s oral spray delivery allows for a much more gentle absorption rate, thus maximising the tolerability of the medicine and increasing the therapeutic window. “If people were to inhale THC-containing materials, their blood levels would rise to 100ng-150ng/ml in about six to eight minutes,” says Guy. “With a standard 10mg, four spray dose of THC by Sativex, you would find that the levels rise to about 3ng or 4ng/ml in two hours.”
Interestingly, the CBD in Sativex also plays a part in tempering the psychoactive effects of THC. “The CBD component decreases the central and psychoactive effects of THC, and therefore makes the THC more tolerable,” says Guy. “That, again, opens the therapeutic window. That’s the essential underlying strategy for developing Sativex, to provide a medicine that captured the pharmacology and therapeutic benefits that were available within cannabis compositions, but to remove the unwanted side effects that would be produced by inappropriate root administration of very high amounts.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataSativex’s UK access troubles
Guy describes Sativex as a potential treatment for MS patients for whom no other treatment has been effective. The drug has been proved to work for just under half of patients who would otherwise have no treatment options left, and whether or not the treatment is working becomes clear in a matter of weeks. Despite this, UK charity the MS Society has reported that some UK MS patients are having trouble getting access to Sativex.
When Sativex received UK approval in the summer of 2010, GW Pharma found that the proportion of NHS primary care trusts (PCTs) willing to reimburse the costs of the drug has remained the same as when it was prescribed as an unlicensed medicine under the named patient basis for the four years before its approval. 85% of PCTs are currently funding Sativex prescriptions, but regulations require that a patient consult with a neurology specialist first. Added to these restrictions is the fact that, especially in the current economic climate, PCTs might be particularly hard to convince when it comes to the cost-effectiveness of a new drug.
Dr Jayne Spink, director of policy and research at the MS Society, would like to see Sativex available to all patients who could potentially benefit from it. “Spasticity is a serious and often distressing symptom of MS that affects at least 20% of people at some point – so new treatments are a welcome development,” she says. “Some people with MS – especially those with more progressive forms – have very few treatment options available. If there is a licensed treatment available that could help, they should be given access to it.”
For Guy, this is an unfortunate side-effect of the UK’s decentralised health system. “There is a postcode lottery in this country,” he says. “It’s very sad because patients who are not getting Sativex are the ones who have very much exhausted all other therapeutic options, and it’s Sativex or nothing.” Now that Sativex has been approved, however, Guy is hopeful that more PCTs will agree to reimburse the drug and a more open atmosphere is established for patients who need it.
Cannabinoid medicine: a whole new world
With Sativex approved in a number of countries and expected to find approval in many more in the coming months, the concept of cannabinoid medicine has been proved as more than a kooky herbal remedy. “The Sativex approval has validated the platform; it says you can make a medicine out of these materials and in this way. So big pharma have said, ‘Fine, that’s great; now we understand’. I think that you will find a lot more interest in the cannabinoid system now that Sativex has been approved.”
So what is the current state of cannabinoid research? As the head of one of the world’s leading cannabinoid research groups, Guy is well-positioned to comment. One of his fundamental tasks is to debunk the myths and preconceptions about this emerging area of science. “We have to take more care to provide far more fundamental data than if we’d just invented a molecule and said, ‘Look, this is what it does, please accept it’. So there is an education process. And it’s very cultural, very different throughout the world. We’ve been operating around the world for ten or 12 years and each country has a very different view on it.”
Another misconception is the overwhelming media focus on the cannabinoid THC, which Guy puts down to the “frisson” of it being the psychoactive component of street cannabis. Indeed, recreational cannabis has been specifically bred to maximise THC at the expense of other cannabinoids. But in terms of research for future applications, Guy describes THC as the least interesting cannabinoid – he even confirms that Sativex will most likely be GW Pharma’s first and last THC-derived treatment. It’s when we take a look at the lesser-known cannabinoids that this research world really begins to open up.
In basic scientific terms, cannabinoids are chemical compounds that interact with and stimulate the cannabinoid receptors that exist in almost all living beings.
These receptors in the body are responsible for modulating other systems in the body. The key is that cannabinoid receptors encourage homeostasis, in other words encouraging the body to do what it is supposed to do.
Guy explains that, unlike with synthetic medicines, which simply trigger a predetermined effect based on the dosage provided, the activation of a cannabinoid receptor on two different occasions could have the opposite result, because the cannabinoid system will always react in a way that most supports homeostasis.
This means that cannabinoids (Guy believes there are more than 100 in existence) share a very good organoleptic and toxic safety profile. But the pharmacology of each cannabinoid differs in the effect that it might bring about. For example, GW Pharma has discovered that THCV, a cannabinoid that is an analogue of THC, acts as to reduce excess activity of the cannabinoid receptor rather than stimulate it. THCV, along with other cannabinoids like CBD, CBDV, CBC and CBG, is forming the bulk of the company’s current research.
“We started a study a few weeks ago in diabetes with a couple of the new cannabinoids,” says Guy, “and we have plans later this year to commence a number of Phase II trials looking at the clinical effects of some of these non-THC cannabinoids.”
In a very real sense, research into the cannabinoid system is developing a new area of scientific thought, not just on environmental substances, but on a fundamental system within the human body, too. Discoveries in this area could hold the key to a new wave of treatment options for patients. “A few years ago, the cannabinoids were referred to as the aspirin of the 21st century,” enthuses Guy. “We have a 20-25-year research programme in front of us. I think that the real benefits of cannabinoid medicines, in terms of human physiology and human pathology, will really come into their own probably ten to 20 years from now.
“My view is that the cannabinoid system will be very much part of modulating and providing homeostasis in humans moving ahead over the next 30-50 years.”
It’s a hugely promising area, although highly complex. It’s an area that will require a huge push from the academic world and the pharmaceutical industry to develop. Fortunately, Guy believes that the world of the cannabinoids is beginning to bloom like never before. “We’re having to develop whole new types of science and whole new ways of assessing what might be going on when you’re introducing a range of cannabinoids,” he says. “Any one of our medicines might have eight to 12 cannabinoids of therapeutic interest in them, each of them working on different mechanisms. We’re spending our time and our money and our resources, ourselves and our partners, to lay down a basis of not only cannabinoid science, but also a very different way of assessing how each of these new cannabinoids are going to have a benefit. So watch this space, I think.”




