To tackle the rising rates of viral hepatitis and its status as a leading cause of death worldwide in the early 2010s, in 2016 governments from every country in the world, coordinated by the World Health Organization (WHO), committed themselves to eliminate viral hepatitis by 2030.
Unfortunately, only 12 countries are currently on track to achieve this aim and viral hepatitis still kills more people than human immunodeficiency virus (HIV) and malaria every year despite its preventability.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
However, the aim of the WHO’s annual World Hepatitis Day is to raise awareness of this largely unknown condition, and how it can be prevented and treated. This year World Hepatitis Day will be held on Sunday 28th July, and centred on ‘finding the missing millions’; the WHO estimates that 300 million people have viral hepatitis and are completely unaware of this fact.
What is hepatitis B?
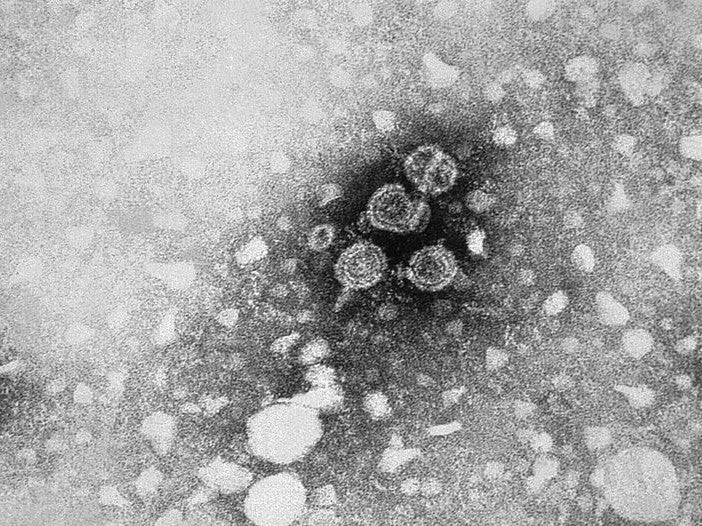
One type of viral hepatitis is hepatitis b virus (HBV), which causes the infectious liver disease Hepatitis B, which, in turn, can develop into a chronic infection. There are an estimated 350 million chronic HBV carriers globally.
The virus also increases the risk of death from cirrhosis and liver cancer. Although HBV is most prevalent in Africa, the virus has been found my researchers to cause 50% of cases of hepatocellular carcinoma in Europe.
HBV is transmitted by exposure to infectious blood or body fluids; infection usually occurs during childbirth or the early years of childhood.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe main issue with HBV infections is that they often do not cause serious side effects until the patients’ condition has deteriorated and become serious, even potentially life-threatening.
Successful prevention and viral suppression of hepatitis B
To control transmission, the WHO recommends a vaccination is administered within the first day of a child being born. If possible, this is then followed up by two or three more doses. It has been estimated that global coverage reached 84% in 2017 and over one billion doses of the hepatitis b vaccine have been administered worldwide since 1982.
In addition to preventative vaccinations, drugs have been developed to keep the virus under control and stop further damage to the liver; the WHO recommends antiviral medications, such as tenofovir and entecavir, as the most effective treatments to supress HBV, however, due to its mechanism HBV patients, like people with HIV, must continue taking these drugs for life.
Developing effective hepatitis B combination drugs
Researchers from Gwangju Institute of Science and Technology, South Korea, screened almost 1,000 drugs approved by the US Food and Drug Administration (FDA) to identify treatments that could improve the efficacy of tenofovir and entecavir in clearing HBV infections as they helped to target different parts of HBV’s life cycles.
Of 13 potential HBV replication inhibitors identified, only antifungal drug ciclopirox successfully inhibited HBV capsid assembly, which is essential to the HBV’s life cycle, and the secretion of HBV DNA in infected cells, both in vitro and mouse models.
The Korean researchers tested the practicality of combining ciclopirox with either of the anti-virals into one therapeutic agent.
They found that the drugs synergise and also that ciclopirox only occupies three of six possible binding sights on the interface of the HBV core protein, leaving space for the anti-viral.
Further research needs to be done to determine why ciclopirox has a binding site preference, as well as focus on developing drugs that can target another crucial part of HBV’s structure and life cycle, the stable covalently closed circular DNA of the HBV.
This feature of the virus is, according to the Gwangju researchers, what prevents the two anti-viral agents from curing HBV completely.


