The US Food and Drug Administration (FDA) has awarded breakthrough therapy designation to Celgene’s Pomalyst (pomalidomide) in HIV-positive and HIV-negative Kaposi sarcoma.
Pomalyst is an oral, small molecule thalidomide analogue that acts via multiple mechanisms of action. It is one of the company’s immunomodulatory imide drug (IMiD) agents that are being developed as treatments for blood cancer.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Pomalyst is indicated for multiple myeloma patients that received at least two prior therapies and saw disease progression within 60 days.
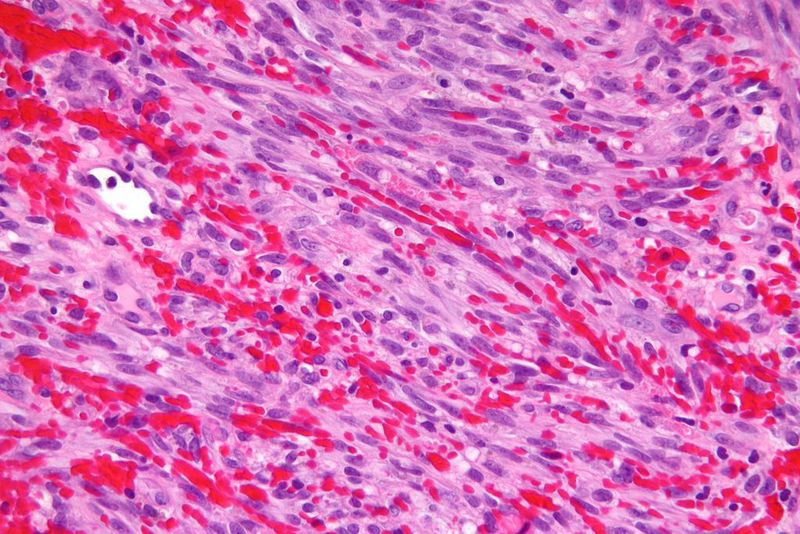
Kaposi sarcoma is a multicentric tumour caused by Kaposi sarcoma-associated herpesvirus or human herpesvirus-8. It is characterised by multiple lesions on the skin and oral mucosa. In some cases, other organs such as the lungs or gastrointestinal mucosa are affected.
The tumour is commonly found in people infected with HIV. Celgene noted that there is a significant need for new treatments due to a lack of approved therapies for HIV-positive patients that are refractory to (or intolerant of) systemic chemotherapy.
Celgene chief medical officer Jay Backstrom said: “The encouraging news of the FDA breakthrough therapy designation for Pomalyst in Kaposi sarcoma reflects the urgency in accelerating the development of therapies to address diseases of this type.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“We will continue to work closely with the agency to move this programme forward for patients with this rare and serious cancer.”
The FDA’s decision was based on findings from a clinical study led by a team in the HIV and AIDS malignancy branch at the Center for Cancer Research of the National Cancer Institutes (NCI).
Celgene intends to conduct two additional clinical studies in HIV-positive and HIV-negative Kaposi sarcoma patients. One of these trials will be conducted in alliance with the AIDS Malignancy Consortium (AMC) to validate and extend data obtained in the NCI study.
AMC will sponsor the second study, which will be performed in Africa.
Celgene is planning to file a supplemental new drug application for Pomalyst in this indication by the end of this year.




