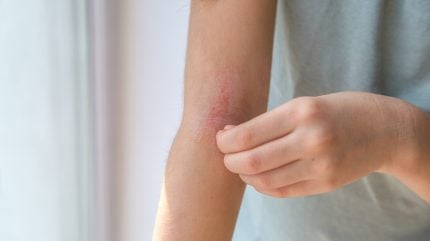
The global atopic dermatitis (AD) market is poised to reach $22.4bn in drug sales within the decade, buoyed by the availability of targeted therapies, market analysis suggests.
A 2025 report published by GlobalData, which analysed the seven major markets of the US, UK, France, Germany, Italy, Spain, and Japan, forecasts sector sales will reach the $22.4bn figure by 2033, up from $8.5bn in 2023, reflecting a compound annual growth rate (CAGR) of 10.2%. While not as fast-growing as the weight loss market in the metabolic disease space, the AD market growth is salient given the market’s size compared to other dermatology indications.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
GlobalData healthcare analyst, Filippos Maniatis, comments: “AD is a growing market with an impressive pipeline of new products from current and future players in the field. The AD space was previously dominated by broad-acting immunomodulatory agents, which are now being slowly replaced by more targeted agents. This shift is likely due to better comprehension of the pathophysiology behind AD and the approval of several new systemic agents.”
GlobalData is the parent company of Pharmaceutical Technology.
One of those targeted agents that has taken the market by storm is Regeneron and Sanofi’s jointly developed Dupixent (dupilumab), first approved in the US in 2017. The blockbuster monoclonal antibody has seen its sales increase year-over-year amid high uptake from AD – reaching $14.9bn in sales in 2024. Dupixent is approved for a range of diseases, including a lucrative asthma indication.
The report says that the gap of unmet need in AD, which is closing courtesy of Sanofi and Regeneron, is set to shrink even further depending on future approvals for more targeted therapies.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataOne modality poised as a future disruptor is the OX40 inhibitor class of drugs that work by inhibiting T-cell activation and inflammation via the OX40 receptor on the immune cells. Pipeline candidates in development include Amgen and Kyowa Kirin’s rocatinlimab, Sanofi’s amlitelimab, and Astria Therapeutics’ telazrolimab.
Another class with a potential future in AD is interleukin (IL) inhibitors. LEO Pharma, GSK, and Nektar all have assets in clinical development.
Maniatis concludes: “With multiple pipeline agents in development, key unmet needs may be further addressed. Such unmet needs include the lack of personalised treatments through improved diagnostic methods, the high cost of current therapy options, the limited therapeutic options for chronic hand eczema, and better long-term disease control and management.”




