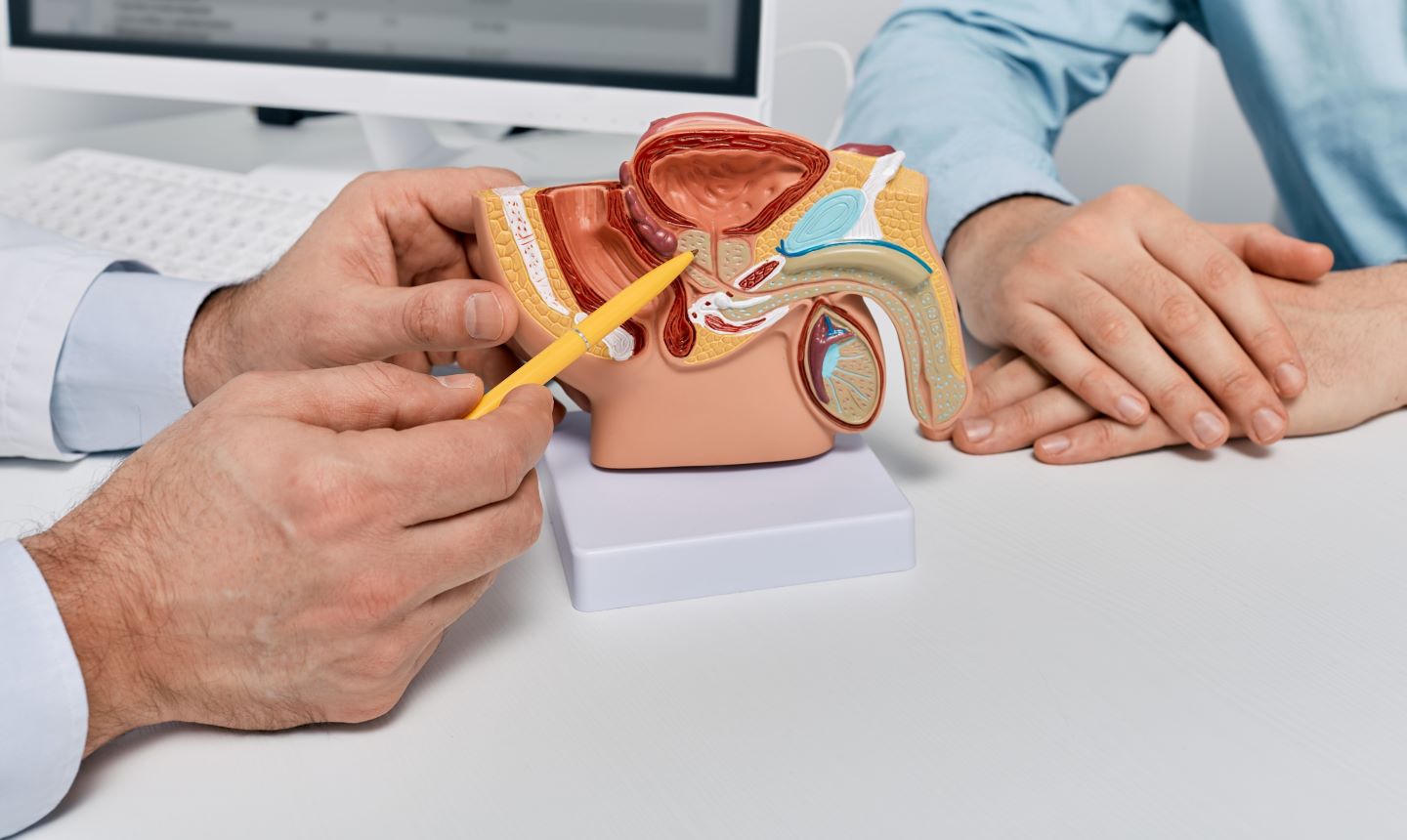
The US Food and Drug Administration (FDA) has awarded fast track designation for Ambrx Biopharma’s ARX517 to treat metastatic castration-resistant prostate cancer (mCRPC).
The investigational therapy is an anti-PSMA [human prostate-specific membrane antigen] antibody-drug conjugate (ADC) intended for patients who advance on an androgen receptor pathway inhibitor.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Fast track status aids in the development and expedites the review process of drugs for serious conditions with unmet medical needs.
The ADC is being evaluated in the first-in-human, open-label Phase I/II APEX-01 trial in mCRPC patients.
This is the only trial underway in the US analysing an ADC that acts on PSMA.
The dose escalation and dose expansion study is enrolling patients with tumours that have advanced following treatment with a minimum of two FDA-approved prostate cancer therapies.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThese participants must have PSA progression defined by at least two rising PSA values, radiographic progression based on RECIST v 1.1, or progression of condition based on the presence of new bone lesions.
Ambrx Biopharma CEO Daniel O’Connor stated: “This fast track designation represents a significant milestone for Ambrx.
“Enabled by our expanded genetic code site-specific conjugation technology, ARX517 has the potential to be a more effective and tolerable treatment option for these patients.”
The company is planning to present updated preliminary data from APEX-01 at a major medical meeting later this year.




