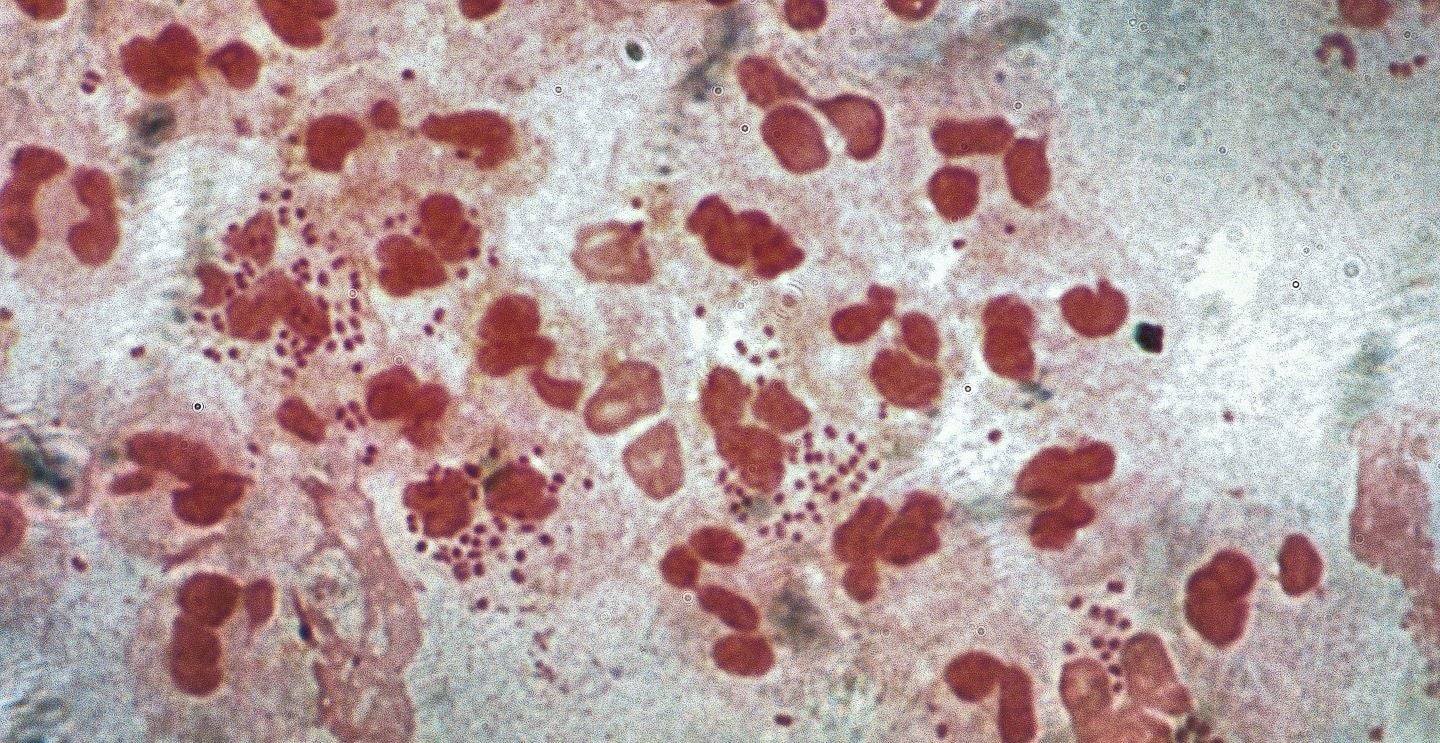
GlaxoSmithKline (GSK) has received a fast track designation for its Neisseria gonorrhoeae investigational vaccine (NgG) from the US Food and Drug Administration (FDA).
The vaccine candidate for gonorrhoea is currently being analysed in a Phase II clinical trial, evaluating its efficacy in healthy adult participants aged 18 to 50 years.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Fast track status supports the development and accelerates the review of new preventive therapies or treatments for serious ailments with unmet medical needs.
GSK vaccines research and development global head Phil Dormitzer stated: “We welcome the FDA’s decision to grant fast track designation for our new vaccine candidate against Neisseria gonorrhoeae infection.
“This designation recognises the potential for a vaccine that could help protect millions of people across the world against the serious health consequences of infection with a bacterium that is considered a high-priority pathogen by the World Health Organisation.”
Neisseria gonorrhoeae bacteria cause gonorrhoea, a sexually transmitted infection. No vaccines are currently approved anywhere in the world.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe latest development comes after the Japanese Ministry of Health, Labour and Welfare (MHLW) approved a renewed indication of GSK’s shingles (herpes zoster) vaccine, Shingrix (recombinant zoster vaccine, adjuvanted).




