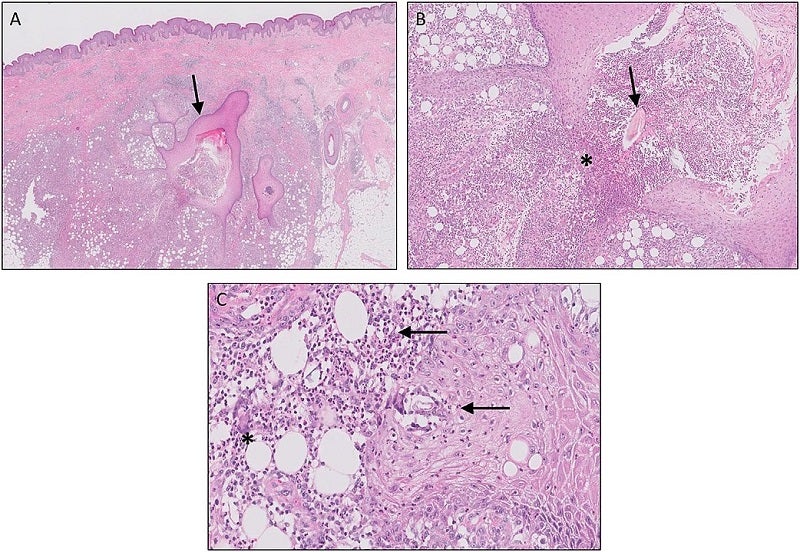
The US Food and Drug Administration (FDA) has granted Fast Track designation for UNION therapeutics’ oral orismilast to treat moderate-to-severe hidradenitis suppurativa (HS).
The company stated that the designation for orismilast highlights the urgent requirement to treat HS, a chronic, inflammatory, progressive, and scarring skin disease.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Orismilast, a PDE4 inhibitor, targets the PDE4 subtypes which are connected to inflammation.
With broad anti-inflammatory properties, the PDE4 inhibitor is also being developed to orally treat psoriasis and atopic dermatitis (AD).
It has also received a Fast Track designation for the treatment of moderate to severe AD.
UNION therapeutics CEO Kim Kjøller said: “We are very pleased to receive this Fast Track designation for oral orismilast in hidradenitis suppurativa (HS) and look forward to working closely with the FDA in the design of the clinical development programme for oral orismilast.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“Our ambition is to provide an effective oral treatment for HS and, in collaboration with dermatologists and patients, develop relevant trial designs, addressing the true needs of patients living with HS.”
The company intends to discuss with the FDA the most suitable endpoints, and target disease severity, along with further steps in oral orismilast’s clinical development to treat HS.
The ongoing investigator-led Phase IIa OSIRIS study of oral orismilast to treat mild to severe HS patients received a treatment extension from the Danish Medicines Agency and Ethics Committee in September last year.
UNION therapeutics said that the study participants who have completed the initial treatment period might continue to receive orismilast MR tablets for 52 weeks.




