FibroGen is set to receive a non-dilutive term loan facility worth up to $150m from funds managed by Morgan Stanley Tactical Value (MSTV) to advance pamrevlumab towards commercialisation.
The loan also provides capital to sustain the development of the company’s pre-clinical pipeline and will be made accessible to the company in three tranches.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
FibroGen will receive an initial tranche of $75m in May 2023. The second tranche of $37.5m will be provided during the third quarter of 2023.
MSTV then has the option to allocate funds for a third tranche, with a maximum potential amount of $37.5m in the same quarter.
FibroGen CEO Enrique Conterno stated: “With the current momentum across our development programmes, this financing strengthens our balance sheet.
“Part of the proceeds will be used to fund our wholly owned pamrevlumab programme as we anticipate five Phase III readouts to occur before mid-2024.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
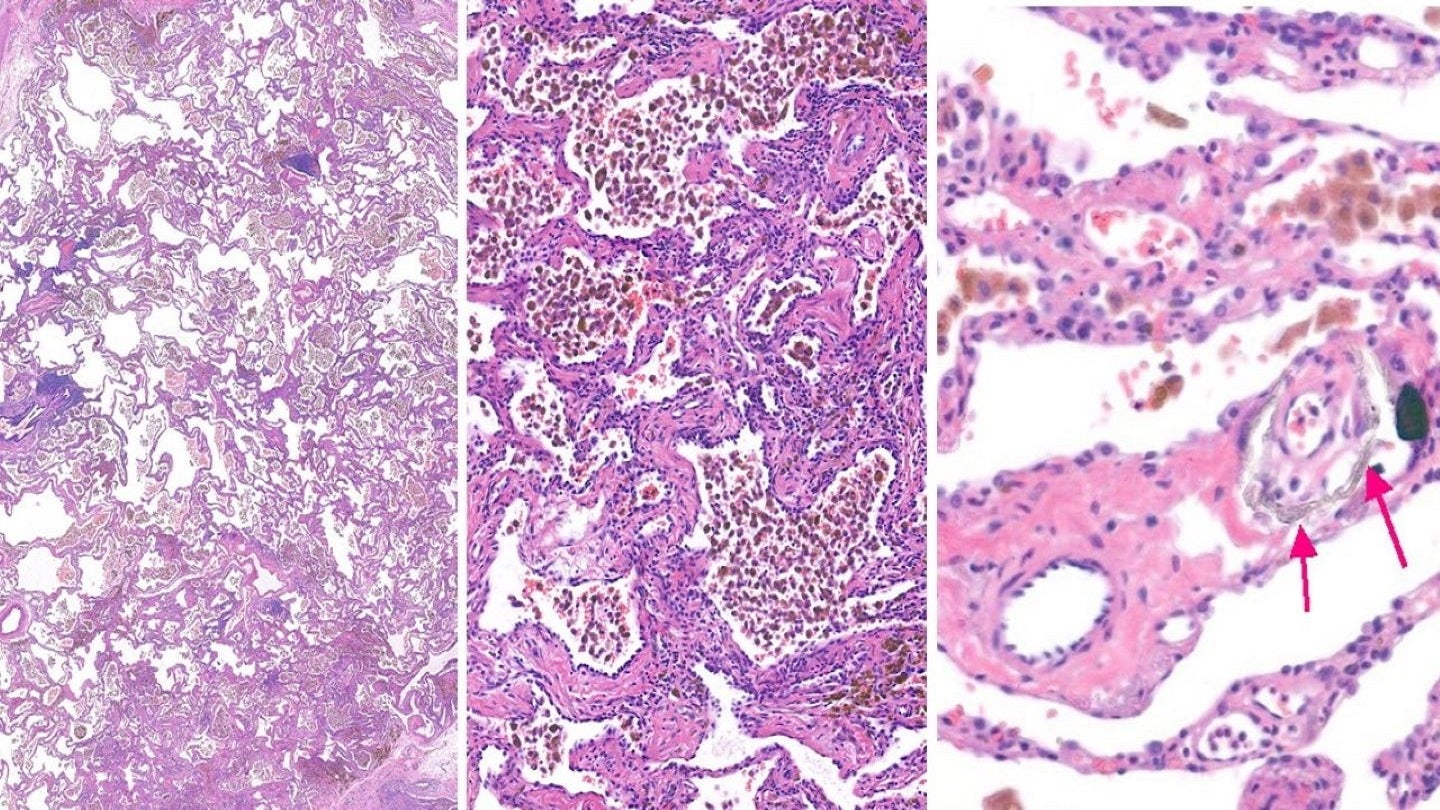
By GlobalDataPamrevlumab, a human monoclonal antibody targeting connective tissue growth factor, is currently undergoing clinical trials for the treatment of conditions including idiopathic pulmonary fibrosis, locally advanced unresectable pancreatic cancer, metastatic pancreatic cancer and Duchenne muscular dystrophy.
The company’s Roxadustat (EVRENZO) has received approval to treat anaemia in chronic kidney disease patients in several countries, including China, Europe, Japan and numerous other nations.
It is undergoing Phase III clinical trials in the US and Europe for treating anaemia associated with myelodysplastic syndromes (MDS) and is in Phase III clinical development in China to treat chemotherapy-induced anaemia.




