Australian pharmaceutical company Incannex has appointed Quest Pharmaceutical Services (QPS) to advance CannQuit-N (Nicotine), CannQuit-O (Opioid) and Renecann Products in the USA and the European Union (EU).
QPS was established in 1995 to provide bioanalytical LC-MS/MS contract services.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The company will provide regulatory advice to Incannex, and manage clinical trials to develop CannQuit and ReneCann products to treat addiction and immune-disordered skin diseases.
QPS is preparing the pre-investigational new drug applications to be submitted to the US Food and Drug Administration (FDA) and the EU’s European Medicines Agency (EMA) for the CannQuit and ReneCann products.
The company will help in managing the clinical trials of the products once the regulators provide approvals for the proposed research and development programmes.
Incannex Healthcare CEO and managing director Joel Latham said: “Not only will QPS assist us with conducting clinical research, but it has also been engaged to advise upon the quickest route to commercialising the products in different regulatory jurisdictions.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn 2022, the company acquired CannQuit and ReneCann products as part of the acquisition of APIRx Pharmaceuticals.
CannQuit-Nicotine combines nicotine and cannabidiol (CBD) in a controlled-release, pharmaceutical-grade medicated chewing gum formulation.
CannQuit-Opioid, named CheWell, combines CBD and an opioid antagonist/agonist in a water-soluble chewable tablet to treat opioid addiction.
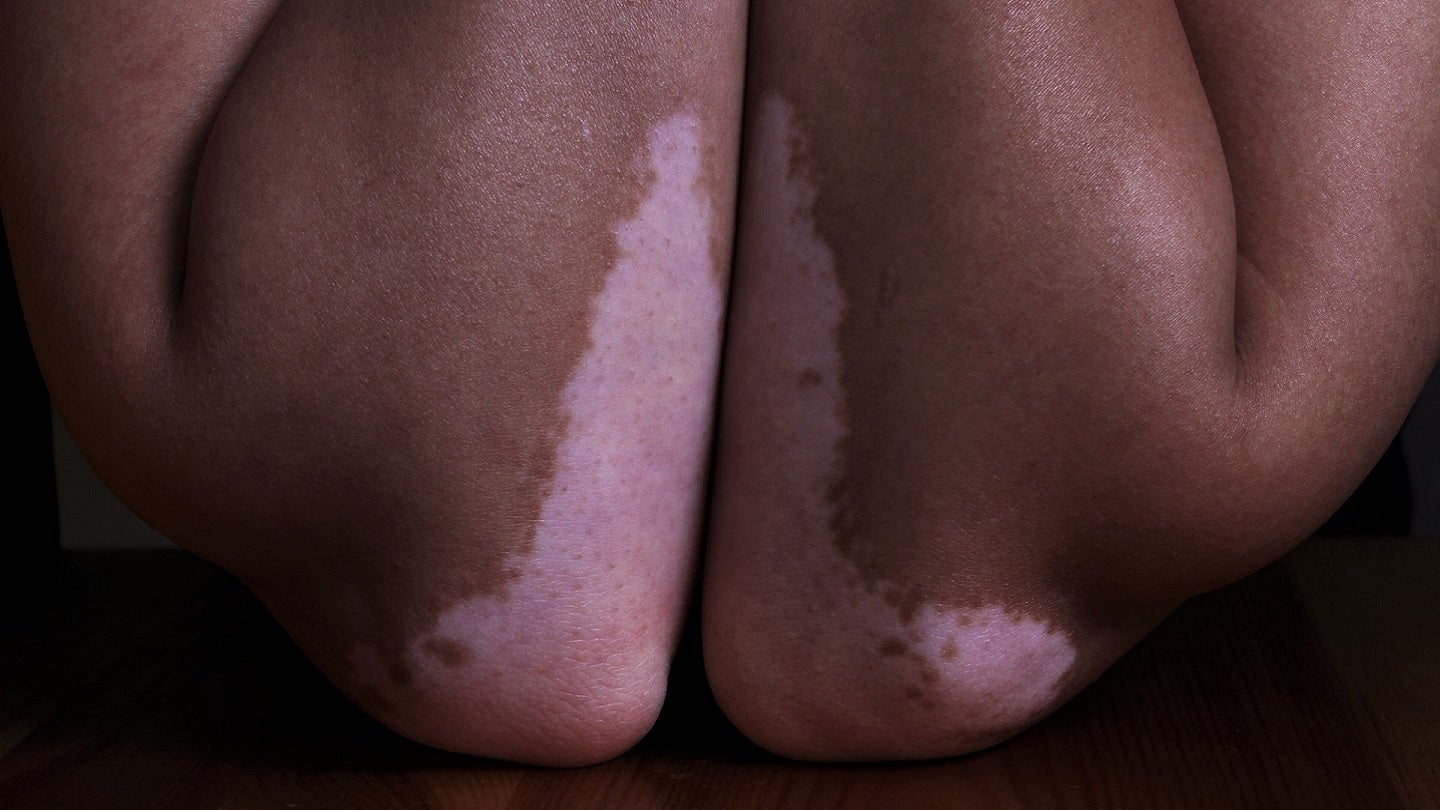
ReneCann is Incannex’s topical cannabinoid formulation and is being developed to treat dermatological conditions caused by immune system disorders, including atopic dermatitis, psoriasis and vitiligo.
French company Eurofins Scientific is currently developing and manufacturing both products. It is also responsible for collecting data regarding the quality and stability of these products.




