
The China Food and Drug Administration (CFDA) has approved Gilead Sciences’ Sovaldi (sofosbuvir at 400mg) for the treatment of patients with chronic hepatitis C virus (HCV) infection.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Sovaldi has been approved for adults, as well as adolescents between 12 and 18 years of age infected with HCV genotype 1, 2, 3, 4, 5 or 6 as a component of a combination antiviral treatment regimen.
As a once-daily oral nucleotide analogue polymerase inhibitor, sovaldi is Gilead Sciences’ first HCV medicine approved in China.
The CFDA approval is supported by a Phase III trial conducted in China that studied sovaldi in combination with ribavirin (RBV) or pegylated interferon plus ribavirin (PegIFN+RBV) in a wide range of difficult-to-cure patients, including treatment-experienced patients and those with compensated cirrhosis.
Gilead Sciences president and chief executive officer Dr John F. Milligan said: “With the approval of Sovaldi, there is now the potential opportunity to transform treatment for HCV patients in China.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“Medicines are one part of the solution but, as we have seen in other countries around the world, there are many other challenges that impact diagnosis, linkage to care and treatment.
“Gilead is committed to working with the government and other stakeholders with the goal to help reduce the significant burden of HCV disease in China.”
The therapy received marketing approval from the US Food and Drug Administration (FDA) in 2013 and from the European Commission in 2014.
The treatment is approved for use in 79 countries, including Australia, India, Indonesia, the Philippines, New Zealand, Canada, Egypt, Switzerland and Turkey.
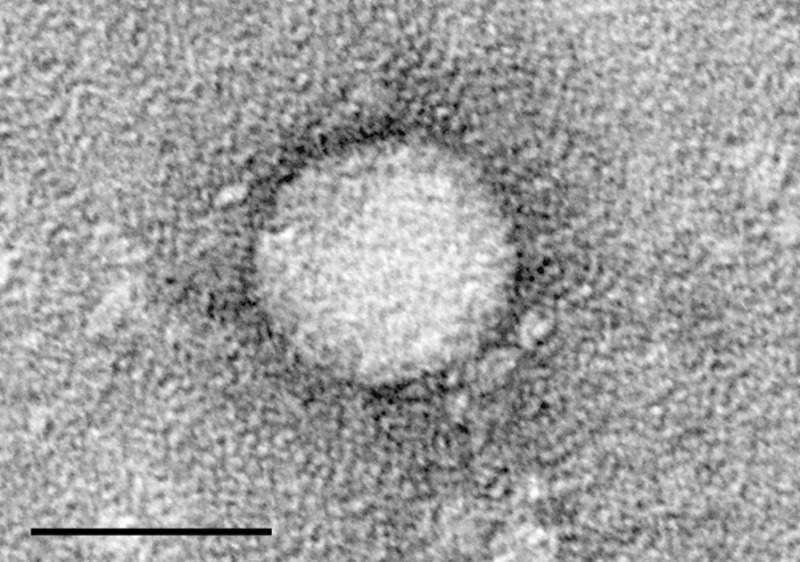
Image: Electron micrographs of hepatitis C virus purified from cell culture. Photo: courtesy of Charles Rice / The Center for the Study of Hepatitis C, The Rockefeller University.




