
Janssen Biotech has entered a worldwide exclusive licence and collaboration agreement with biopharmaceutical company Protagonist Therapeutics for the development, manufacture, and commercialisation of PTG-200 for the treatment of inflammatory bowel disease (IBD).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
PTG-200 is an oral interleukin-23 receptor (IL-23R) antagonist drug candidate that can be used to treat patients suffering from IBD, Crohn's disease and ulcerative colitis (UC).
Under the deal, Protagonist Therapeutics will receive an upfront payment and is eligible to receive development and commercialisation milestone payments.
As a potential first-in-class oral IL-23R antagonist, PTG-200 is currently in Investigational New Drug (IND) enabling studies.
Janssen IBD disease area leader and vice-president Dr Scott Plevy said: “We look to continue to pioneer the science of inflammatory bowel disease and advance novel therapeutics like PTG-200, an oral therapy that targets a validated pathway and acts locally in the gut at the site of disease.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“We're particularly excited to add an oral peptide-based therapy to our robust portfolio as we aim to address the increasing incidence of inflammatory bowel disease and the growing needs of people living with Crohn's disease and ulcerative colitis around the world.”
A Phase I clinical trial for PTG-200 is slated to begin later this year.
According to a new report issued by the Centers for Disease Control and Prevention, IBD currently affects five million people worldwide and the incidence is rising with a near three-time increase in the US.
The transaction is expected to conclude in the third quarter of this year.
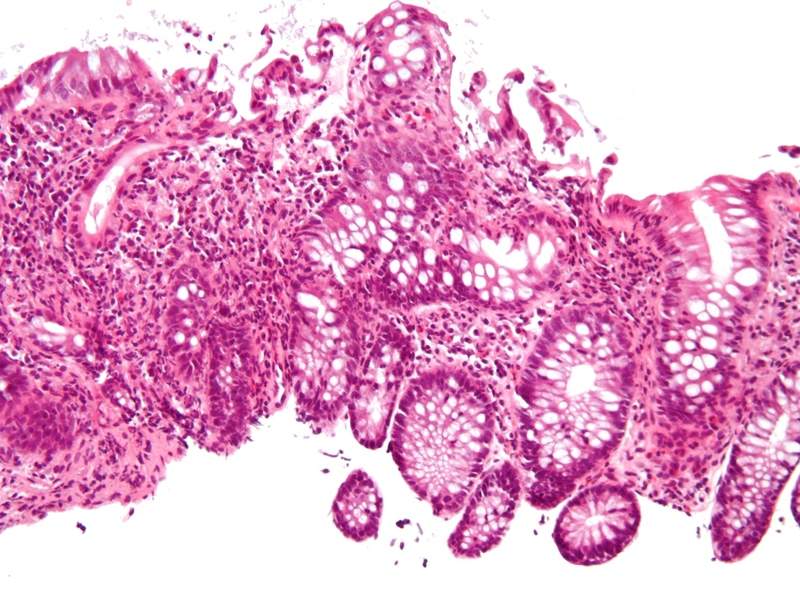
Image: Intermediate magnification micrograph of cryptitis in a case of Crohn's disease. H&E stain. Photo: courtesy of Nephron / Wikipedia.




