
Janssen Sciences Ireland has taken a strategic decision to discontinue further development of its investigational regimen JNJ-4178 for the treatment of patients with hepatitis C.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
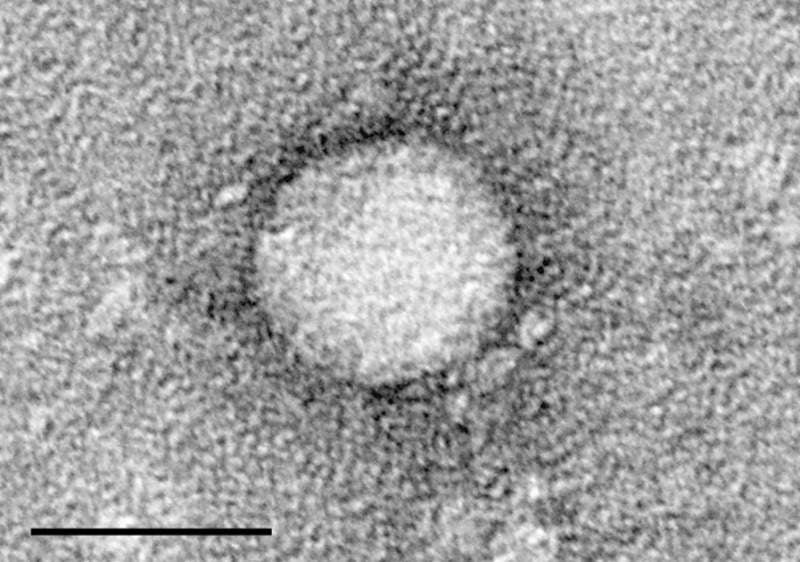
Hepatitis C is an infectious disease caused by the hepatitis C virus (HCV) and commonly affects the liver.
JNJ-4178 is a combination of three directly acting antivirals, AL-335, odalasvir and simeprevir.
Though the company will complete the ongoing Phase II studies for JNJ-4178, the treatment will not undergo any further development thereafter.
Janssen Sciences Ireland has made the decision owing to the growing availability of a wide range of highly effective and approved treatment options for hepatitis C patients.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataJanssen Infectious Disease Therapeutics global therapeutic area head Dr Lawrence Blatt said: “Going forward, our hepatitis research and development (R&D) efforts will focus on chronic hepatitis B, where a high unmet medical need still exists.
“Our scientists are energised by this challenge and our research ambition is to achieve a functional cure for hepatitis B which affects over a quarter of a billion people globally.
“At Janssen, we focus our research and development on areas of greatest unmet medical need where we can combine our excellent internal science with the best available external innovation to bring optimised solutions and maximum benefit to patients.”
Nearly a decade ago, Janssen co-developed telaprevir, which is a first-in-class protease inhibitor used in combination therapy to treat patients with chronic HCV.
Subsequently, the company, in collaboration with Medivir, developed and launched the second-generation protease inhibitor Olysio (simeprevir), which is currently approved in several countries worldwide.
Image: Electron micrographs of hepatitis C virus purified from cell culture. Photo: courtesy of TimVickers.




