Swiss Biotech firm Novimmune has entered into an option and licence agreement with Roche company Genentech to develop and commercialise its anti-toll-like receptor 4 (TLR4) monoclonal antibody, NI-0101, to treat rheumatoid arthritis and other auto-immune diseases.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Financial details have not been disclosed. The agreement follows a deal signed between the two companies in July 2010 to discover and develop antibodies that target the IL-17 signalling pathway for the treatment of auto-immune diseases.
The results of a Phase IIa proof-of-concept study to be performed by Novimmune in patients with rheumatoid arthritis are yet to be reported.
Novimmune chairman and chief executive officer Eduard Holdener said: "We are excited to have now signed a second agreement with Genentech to develop a medicine for rheumatoid arthritis, where there is significant unmet need and the potential to become the first personalised medicine in rheumatoid arthritis."
Under the exclusive agreement, Genentech has an option to licence all rights to develop and commercialise the drug.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIf the licence option is exercised, Genentech would be undertaking further development and potential commercialisation of the drug and would pay Novimmune an undisclosed amount.
In addition, upon obtaining certain predetermined clinical, regulatory and commercial benchmarks, Novimmune would be eligible for payments and royalties on net sales achieved through the licence agreement.
Novimmune develops antibody-based drugs to treat inflammatory diseases, immune-related disorders, and cancer.
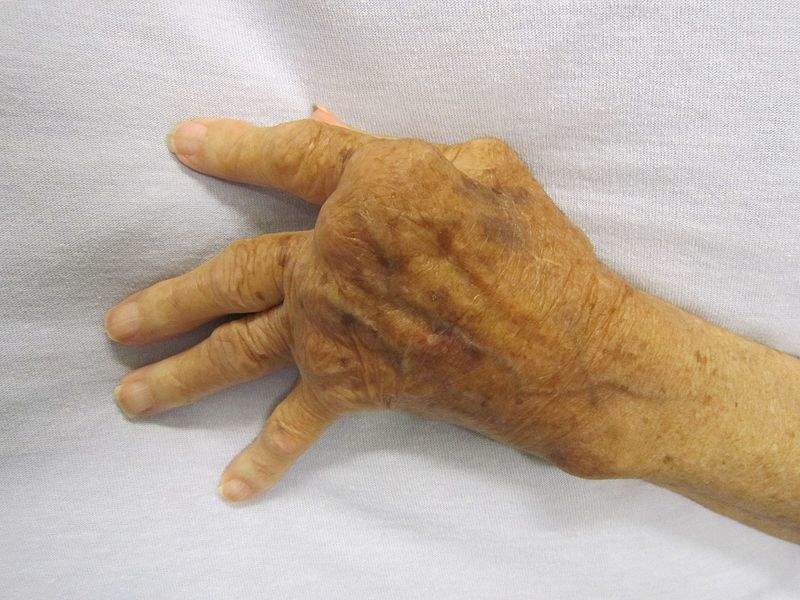
Image: Severe arthritis in hand that was not treated. Photo: courtesy of James Heilman, MD.




