
Swiss-based biopharmaceutical company Prexton Therapeutics has closed a Series B financing round of €29m to finance two Phase II studies of its lead product, Foliglurax, in Parkinson’s disease (PD).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
PD is a devastating progressive neurological condition that affects around 6.3 million people globally.
The financing round is co-led by Forbion Capital Partners (NL) and Seroba Life Sciences (IE) and includes existing investors Merck Ventures (NL), Ysios Capital (ES) and Sunstone Capital (DK).
Prexton Therapeutics CEO Francois Conquet said: “It is a testament to the potential of Foliglurax that we have successfully completed such a significant funding round from high-quality investors.
“We are now keen to begin our Phase II efficacy trials and continue the development of Foliglurax as a potential new therapeutic for Parkinson’s disease.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataSet to start this year, the trials will take place in specialist centres within Europe and the US.
PD is caused by the degeneration of dopaminergic brain cells and its main symptoms are resting tremour, muscle rigidity ('OFF-time') and uncontrolled movements ('Dyskinesia').
Prexton aims to stimulate a compensatory neuronal system that is unaffected by PD.
Foliglurax activates a specific target of the glutamatergic system (mGluR4) instead of targeting the dopaminergic system, to treat the motor symptoms of PD.
In September last year, Prexton completed a Phase I trial with Foliglurax and the results proved Foliglurax as safe and well-tolerated at doses well above those that produce robust effects in PD primate models.
PD is more prevalent in people above 60 and its incidence is expected to increase as the average age of the population increases.
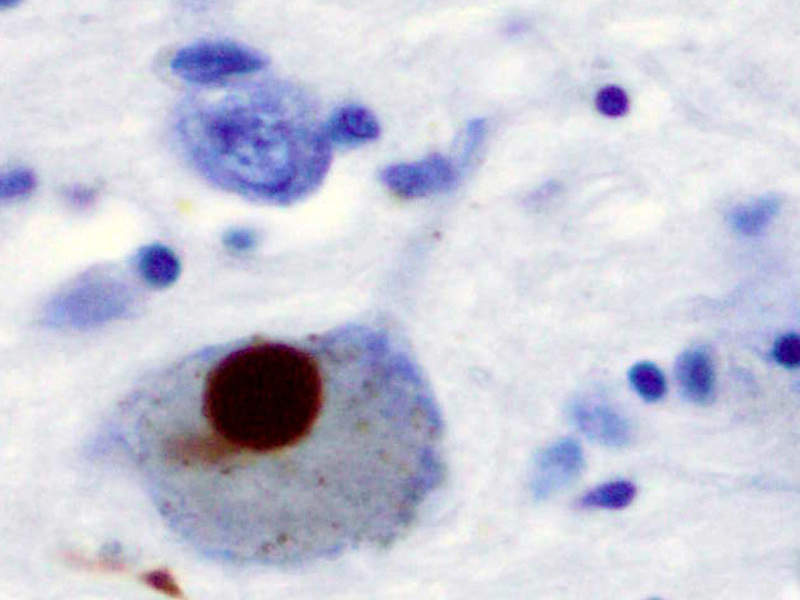
Image: Immunohistochemistry for alpha-synuclein showing positive staining (brown) of an intraneural Lewy-body in the Substantia nigra in Parkinson's disease. Photo: courtesy of Marvin 101.




