
Teijin Pharma, the core company of the Teijin Group’s healthcare business, has entered a new licence agreement with Merck to develop, manufacture and commercialise an investigational preclinical antibody candidate targeting tau protein.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Changes in tau protein are related to a wide range of diseases of the nervous system, including Alzheimer’s disease.
Teijin Pharma president Akihisa Nabeshima said: “We believe that Merck’s strong neuroscience expertise makes it well suited to maximise the potential of this candidate.”
The primary causes of Alzheimer’s disease are considered to be two pathological changes in the brain, senile plaques caused due to the extracellular deposition of amyloid β peptide, and neurofibrillary tangles, which is a result of intracellular accumulation of hyperphosphorylated tau proteins.
Under the agreement, Merck will have exclusive global rights to develop, manufacture and commercialise the anti-tau antibody.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe antibody developed by Teijin Pharma is designed to bind to hyperphosphorylated tau proteins and will be evaluated to test its ability to inhibit the progression of symptoms of dementia.
Merck research laboratories neuroscience discovery vice-president Darryle Schoepp said: “Teijin Pharma scientists have made important progress to advance this investigational anti-tau antibody to this stage of development.
“Merck remains committed to developing meaningful therapeutic options for the treatment of Alzheimer’s and other neurological diseases.”
Headquartered in Kenilworth, New Jersey, US, the global biopharmaceutical company will in exchange make an upfront payment to Teijin Pharma, which is also eligible to receive development, regulatory and sales milestone payments.
Teijin Pharma will also receive royalties on product sales and retain an option to co-promote an approved product in Japan.
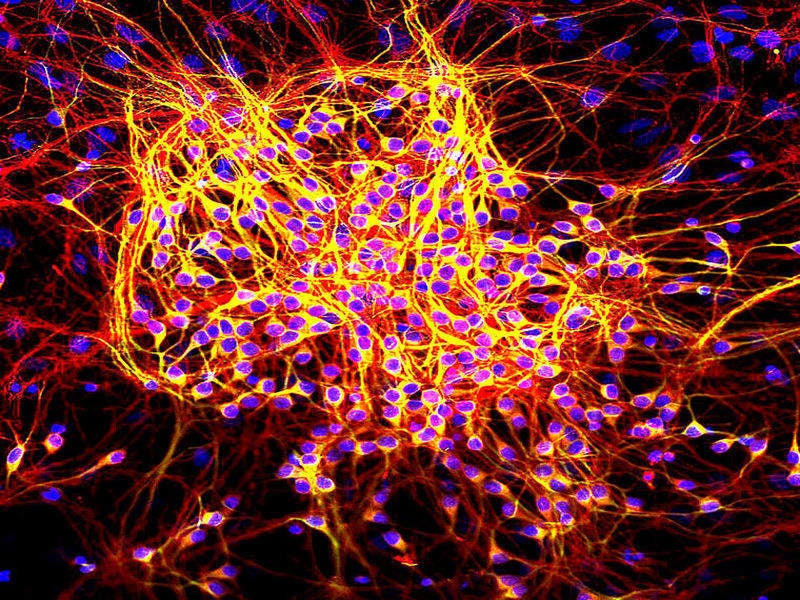
Image: Neurons were grown in tissue culture and stained with antibody to MAP2 protein in green and MAP-tau in red using the immunofluorescence technique. Photo: courtesy of GerryShaw / Wikipedia.




