
The US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have granted orphan drug designation to Therachon’s TA-46 therapy for treating patients with achondroplasia.
Therachon is a global biotechnology company that focuses on the development of rare genetic diseases.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
A common form of short-limbed dwarfism, achondroplasia is a rare, genetic condition that affects approximately one in 15,000 children.
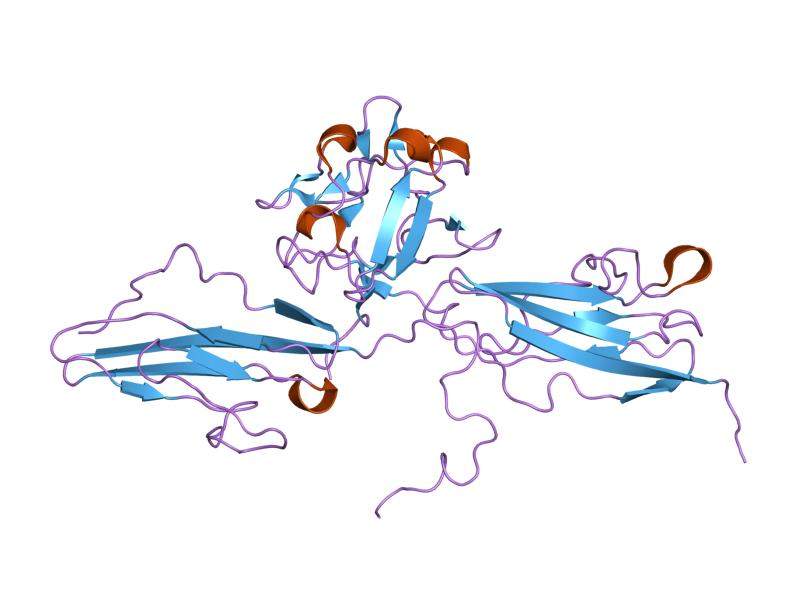
The condition is caused by a genetic mutation of the fibroblast growth factor receptor 3 (FGFR3), which hinders the growth of child bone.
Achondroplasia is also accompanied by life-altering neurological and orthopedic ear, nose and throat complications.
The only treatment currently available for the condition is limb-lengthening surgery, which is an extremely invasive surgical procedure that resolves the issue of height but fails to address the specific achondroplasia-associated complications.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataTherachon chief executive officer Dr Luca Santarelli said: “The granting of orphan drug designation by both regulatory bodies highlights the global unmet medical need for new therapies for patients with achondroplasia.
“We are committed to translating the promise of TA-46 into a novel medicine for children with achondroplasia and look forward to start our clinical programme later this year.”
Therachon’s TA-46 helps modulate the function of the FGFR3 directly.
The mutations of FGFR3 that cause achondroplasia result in excessive activation of this receptor, which slow down normal growth of the bone.
TA-46 helps prevent the excessive activation of FGFR3 by binding to its natural ligands and prohibiting them from over-activating the mutated FGFR3.
Image: Cartoon representation of the molecular structure of FGFR3 protein registered with 1ry7 code. Photo: courtesy of Jawahar Swaminathan and MSD staff at the European Bioinformatics Institute.




