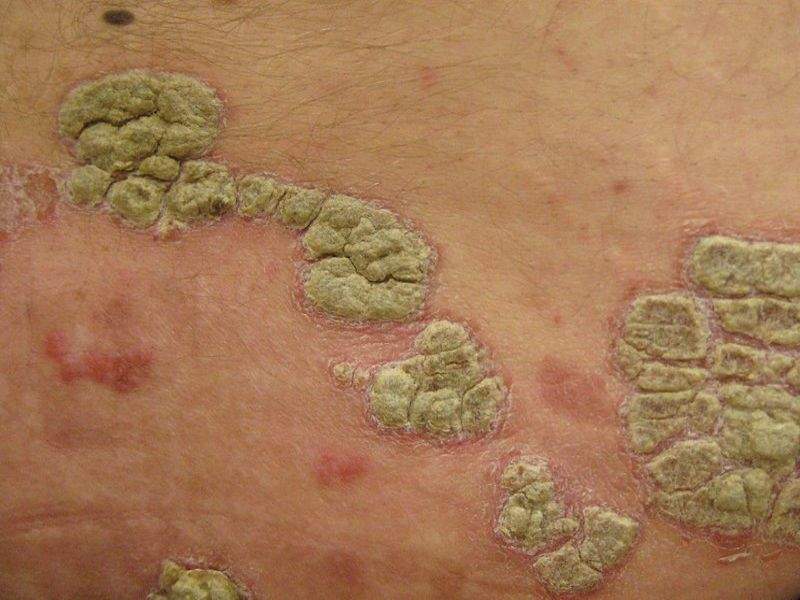
The US Food and Drug Administration (FDA) has approved Siliq (brodalumab) to treat adults with moderate-to-severe plaque psoriasis, a skin condition that causes patches of skin redness and flaking.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Valeant Pharmaceuticals is marketing the drug Siliq, which is administered as an injection and used to treat patients who are candidates for systemic therapy or phototherapy and have failed to respond to other systemic therapies.
FDA Center for Drug Evaluation and Research Office of Drug Evaluation III director Julie Beitz said: "Moderate-to-severe plaque psoriasis can cause significant skin irritation and discomfort for patients, and today’s approval provides patients with another treatment option for their psoriasis.
"Patients and their healthcare providers should discuss the benefits and risks of Siliq before considering treatment."
Occurring more commonly in patients with a family history of the disease, Psoriasis most often begins in people between 15 and 35.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataPlaque psoriasis is the most common form of psoriasis, in which patients develop thick, red skin with flaky, silver-white scales.
Siliq’s active ingredient (brodalumab) binds to a protein that causes inflammation and inhibits the inflammatory response that plays a vital role in plaque psoriasis development.
The safety and efficacy of this drug were established in three randomised, placebo-controlled clinical trials that involved a total of 4,373 adult participants with moderate-to-severe plaque psoriasis.
During clinical trials, patients treated with Siliq were observed with suicidal ideation and behaviour, including completed suicides.
Users of Siliq with a history of suicidality or depression reported an increased incidence of suicidal ideation and behaviour compared to users without this history.
The most common adverse reactions reported with the use of this drug are joint pain, headache, fatigue, diarrhoea, throat pain, nausea, muscle pain, injection site reactions, influenza, low white-blood cell count and fungal infections.
Image: Plaques of psoriasis. Photo: courtesy of James Heilman, MD.




