Netherlands-based biotechnology company Pharming Group has submitted a supplemental biologics licence application (BLA) to the US Food and Drug Administration (FDA) for Ruconest (Recombinant Human C1 Esterase Inhibitor / conestat alfa) for prophylaxis of hereditary angioedema (HAE).
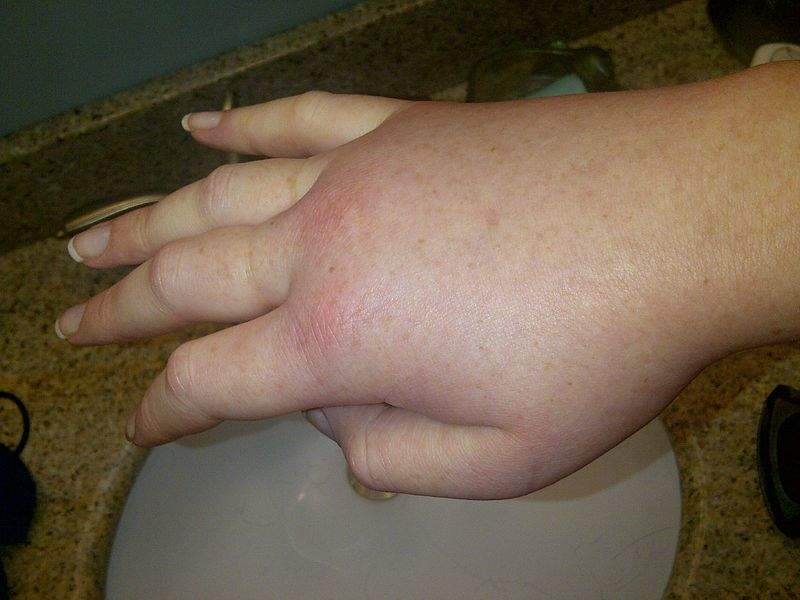
HAE is a rare genetic disorder that causes spontaneous and recurrent episodes of swelling (edema attacks) of the skin in different parts of the body, and in the airways and internal organs.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Ruconest is indicated for use to prevent attacks in adult and adolescent patients with HAE.
Pharming Group chief operations officer Dr Bruno Giannetti said: “HAE patients in the US are currently facing a shortage of plasma-derived C1 inhibitor used to prevent attacks.
“We understand that this supply disruption has had serious consequences for them, including additional stress, disease-related complications, and hospitalisations. We look forward to working with FDA and potentially providing these patients an alternative and plasma free option for HAE prophylaxis.”
The BLA submission is based on data from two completed trials of Ruconest for the prophylaxis of HAE attacks.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe trials involved a randomised, double-blind, placebo-controlled trial and an open-label study on a total of 56 patients and demonstrated consistent efficacy and safety results.
The Pharming product is currently approved for the treatment of acute HAE in Europe, Israel, South Korea and the US.
The company focuses on the development of new products for the safe, effective treatment of rare diseases and unmet medical requirements.




