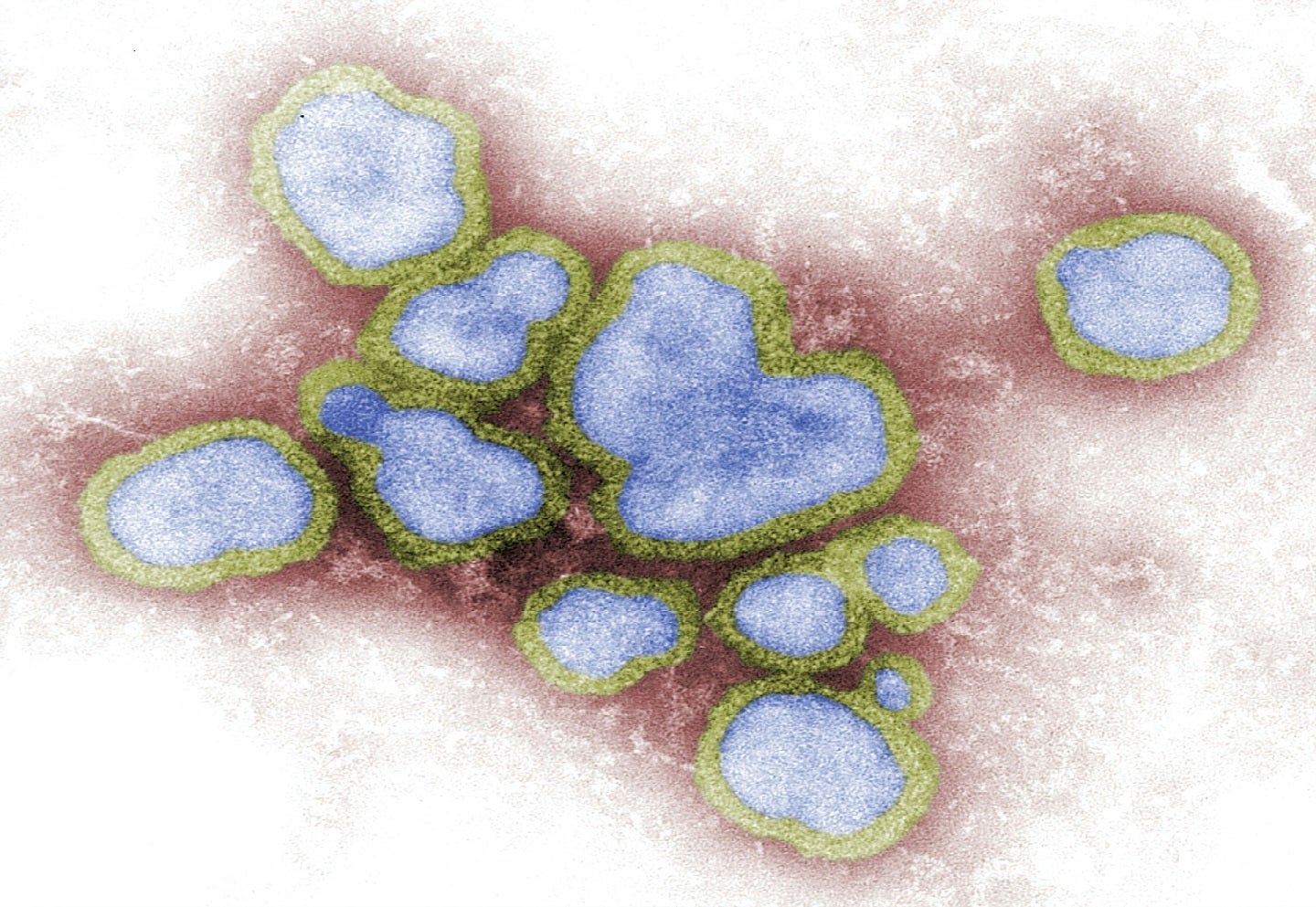
SAB Biotherapeutics has received breakthrough therapy designation (BTD) from the US Food and Drug Administration (FDA) for SAB-176, an investigational immunotherapy to treat influenza.
The newly granted status allows SAB to speed up the development and review of SAB-176, which is currently being studied for use in post-exposure prophylaxis for Type A and Type B influenza in high-risk patients, including those with anti-viral resistant strains.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It also confirms that the multi-epitope targeting modality of SAB-176 is different from that of monoclonal antibodies (mAb) that bind to a single epitope.
The status further confirms the treatment’s capability to sustain its efficacy over viral mutations as well as to avert or minimise the risk of new treatment-resistant influenza strains.
SAB-176 received fast-track designation from FDA in mid-April 2023.
SAB also obtained FDA guidance and regulatory support to accelerate the development of SAB-176 by commencing a Phase llb dose range-finding efficacy and safety study in patients who are highly prone to developing severe disease.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataSAB-176 has already undergone a range of clinical and pre-clinical studies, including a Phase l trial in healthy subjects and a Phase lla challenge study, concluded last year.
SAB Biotherapeutics co-founder, president and CEO Eddie Sullivan stated: “Influenza continues to pose considerable health concerns both in the US and on a global scale. This breakthrough therapy designation signifies an important step forward in our fight against this disease.
“Even though both designations can be requested early in development, the requirements for breakthrough therapy designation are higher than those for the fast track programme.
“For breakthrough therapy designation, the improvement demonstrated must be substantial.”




