Switzerland-based Santhera Pharmaceuticals has signed an agreement with Idorsia to acquire the option to exclusively in-license dissociative steroid vamorolone via a sub-license mode in all indications worldwide, except Japan and South Korea.
As per the agreement, Idorsia will receive one million new registered shares from Santhera’s existing authorised share capital and a $20m upfront payment. Of this amount, $15m is meant to compensate Idorsia for its investment into the Phase IIb VISION-Duchenne Muscular Dystrophy (DMD) study, which is currently being undertaken by ReveraGen.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Following this transaction, Idorsia will become the largest shareholder in Santhera with a 13.3% equity.
The shares issued to Idorsia will be put to a lock-up undertaking that expires once vamorolone gets marketing authorisation for DMD in the US.
Santhera may choose to exercise the option after receipt of data from the Phase IIb VISION-DMD study (VBP15-004) and a one-time consideration to Idorsia of $30m.
After the exercise of the global license option by Idorsia and sub-license option by Santhera, the latter will pay regulatory and commercial milestone payments of up to $80m to Idorsia in the DMD indication and four one-time sales milestone payments of up to $130m in total.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataSanthera chief executive officer Thomas Meier said: “This agreement underscores our strategy of in-licensing high-quality, late-stage rare disease assets, which leverage our existing capabilities and expertise. We are also delighted to welcome Idorsia as our largest shareholder and partner and look forward to working with ReveraGen in the development of vamorolone, which has the potential to replace standard glucocorticoids as a treatment for DMD.”
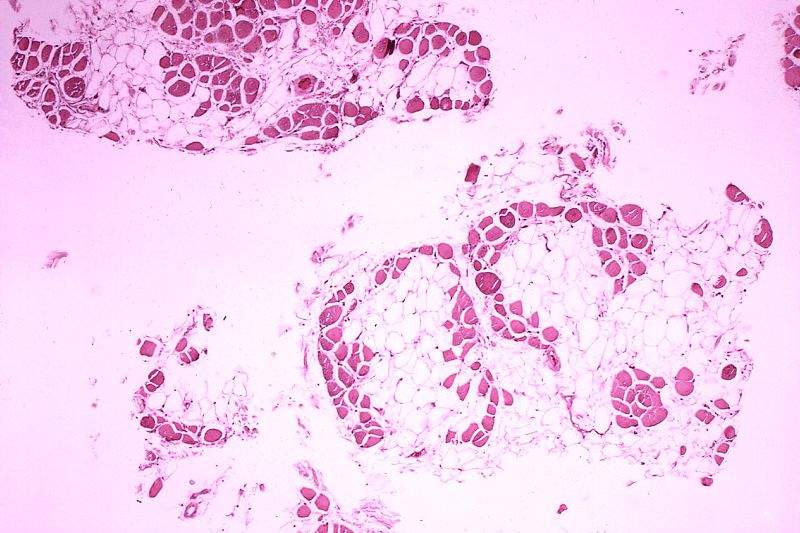
Initial clinical data reports indicate that vamorolone has the anti-inflammatory efficacy of steroids but with reduced safety concerns associated with steroids. This drug will serve as a considerable improvement over the current standard of care glucocorticoid therapy for patients suffering from DMD.
Vamorolone is a first-in-class drug that binds to the same receptors just as glucocorticoids; however, vamorolone differs from the latter as it modifies the downstream activity of the receptors. Glucocorticoids are currently used as the standard of care in children and adolescent patients suffering from DMD.
Discovered by US-based ReveraGen BioPharma, the drug was developed with participation in funding and design of studies by 12 non-profit foundations, the US National Institutes of Health, the US Department of Defense and the European Commission’s Horizon 2020 programme.
In 2016, Actelion secured an option to license the product. Idorsia got this option after Johnson&Johnson acquired Actelion the following year.




