Biotechnology firm Septerna has raised $150m in a Series B funding round to develop its pipeline of new oral small molecule therapies that act on G protein-coupled receptors (GPCRs).
New investor RA Capital Management led the round, with current investors Third Rock Ventures, Invus, Catalio Capital Management, Samsara BioCapital and BVF Partners among others taking part.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
RA Capital Management partner Jake Simson will join the board of directors of Septerna.
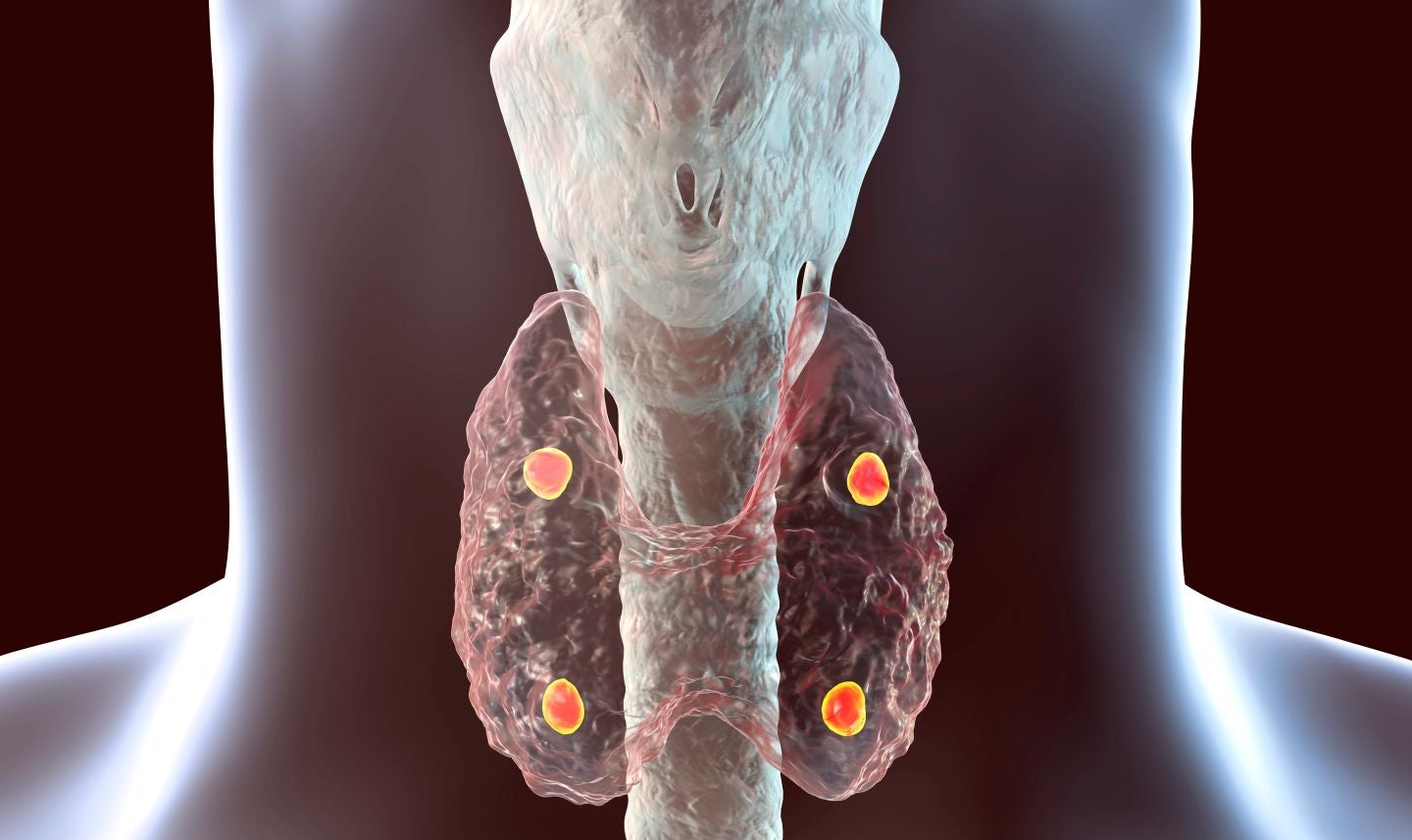
The proceeds will be utilised for portfolio development and to progress its lead parathyroid hormone 1 receptor (PTH1R) programme to clinical proof-of-mechanism.
This PTH1R agonist programme is being developed to treat hypoparathyroidism, a condition marked by reduced levels of PTH.
The company will also use the funds to advance the preclinical development of a second thyroid-stimulating hormone receptor-targeting programme and other initial-stage assets.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataSepterna co-founder and CEO Jeffrey Finer stated: “This milestone marks an important transition for Septerna to a product-development company, with plans to advance our lead PTH1R programme to clinical proof-of-mechanism while building out a multi-product pipeline for a range of diseases.
“This is an exciting time for GPCR drug development, and we are eager to move our novel products toward clinical development.”




